Abstract
ALX-0681 is a therapeutic Nanobody targeting the A1-domain of VWF. It inhibits the interaction between ultra-large VWF and platelet GpIb-IX-V, which plays a crucial role in the pathogenesis of thrombotic thrombocytopenic purpura (TTP). In the present study, we report the efficacy and safety profile of ALX-0681 in a baboon model of acquired TTP. In this model, acute episodes of TTP are induced by administration of an ADAMTS13-inhibiting mAb. ALX-0681 completely prevented the rapid onset of severe thrombocytopenia and schistocytic hemolytic anemia. After induction of TTP, platelet counts also rapidly recovered on administration of ALX-0681. This effect was corroborated by the full neutralization of VWF activity. The schistocytic hemolytic anemia was also halted and partially reversed by ALX-0681 treatment. Brain CT scans and post mortem analysis did not reveal any sign of bleeding, suggesting that complete neutralization of VWF by ALX-0681 under conditions of thrombocytopenia was not linked with an excessive bleeding risk. The results obtained in this study demonstrate that ALX-0681 can successfully treat and prevent the most important hallmarks of acquired TTP without evidence of a severe bleeding risk. Therefore, ALX-0681 offers an attractive new therapeutic option for acquired TTP in the clinical setting.
Introduction
VWF is a large, adhesive glycoprotein circulating in plasma as multimers of up to 20 000 kDa.1,2 It plays a key role in hemostasis, because it recruits circulating platelets to damaged vessels through binding of the VWF A1-domain to the GpIb-IX-V platelet receptors. It also acts as a carrier for factor VIII (FVIII), thereby preventing its proteolytic degradation while inactive.3 VWF is synthesized predominantly by endothelial cells, but also by megakaryocytes, and is secreted as ultra-large VWF multimers (ULVWF; ie, those > 20 000 kDa).4 These ULVWF multimers are abnormally adhesive and able to spontaneously link platelets irrespective of vessel injury.5 In normal circulation, ULVWFs are cleaved by the protease ADAMTS13 into normal-sized multimers (500-20 000 kDa).6,7 The agglutination properties of VWF are therefore reduced, because the GpIb-IX-V platelet receptor binding site in the A1 domain of regular sized VWF is cryptic and only exposed under high shear conditions.8-12
In the condition of thrombotic thrombocytopenic purpura (TTP), there is an inability to process ULVWF because of inhibition (acquired) or dysfunction (congenital) of the protease ADAMTS13.13,14 TTP is a rare but life-threatening disorder characterized by clinical features such as thrombocytopenia, hemolytic anemia, fever, and neurologic/organ dysfunction. All clinical symptoms are associated with excessive platelet aggregation and the formation of platelet-rich thrombi occluding the microcirculation due to the lack of ADAMTS13 activity. Acquired TTP presents both as an acute idiopathic form with no clear underlying cause and as a secondary form that develops in association with other clinical conditions (eg, pregnancy, cancer, HIV, and autoimmune disorders).14,15 The idiopathic form is the predominant form and is related to ADAMTS13 deficiency because of an autoimmune mechanism.
The current primary treatment for acquired TTP is plasma-exchange therapy, which removes the adhesive ULVWF multimers and attached platelets, as well as autoantibodies to ADAMTS13.16,17 Corticosteroids and immunosuppressive therapy are also used in combination with plasma exchange to reduce the production of ADAMTS13 Abs for refractory or relapsing disease.18 Although plasma exchange has significantly reduced mortality rates,19 acquired TTP still carries a considerable risk of mortality and morbidity and new therapeutic approaches are urgently needed to better manage this disorder.19,20 ALX-0681 is a bivalent humanized Nanobody containing 2 identical monovalent building blocks targeting the A1 domain of VWF, as described for ALX-0081.21 Nanobodies are therapeutic proteins derived from the heavy-chain variable domains that occur naturally in heavy-chain-only Igs from Camelidae.22,23 ALX-0081 and ALX-0681 are composed the same active drug targeting VWF, but are denominated according to delivery route as intravenous (IV) and subcutaneous (SC), respectively. For clarity, ALX-0681 is used herein to describe both forms. The antithrombotic Nanobody binds avidly to multimeric VWF and blocks the interaction of any sizes and activation stages of multimeric VWF with the platelet GpIb-IX-V receptor. Given the potential role of VWF-dependent formation of platelet-rich thrombi in the pathogenesis of TTP, the anti-VWF Nanobody may provide an attractive new option for the treatment of acquired TTP. Data on the anti-VWF aptamer ARC1779 from a prematurely terminated phase 2 study in acute TTP patients indeed demonstrated that blocking the A1 domain of VWF has the potential to increase platelet counts in conjunction with plasma-exchange therapy.24,25 Given the well-established role of the VWF-platelet interaction during normal hemostasis, bleeding risk is a relevant safety concern for anti-VWF compounds. Currently available in vivo and in vitro data on ALX-0681, ARC1779, and other anti-VWF antagonists have not demonstrated a significant impact of functional VWF neutralization on bleeding tendency,26,27 but the effect of VWF inhibition in situations of low platelet counts has not been assessed thoroughly.
A baboon model mimicking the acute early episode of acquired TTP, in which the disease was induced by administration of an ADAMTS13-inhibiting monoclonal antibody (mAb) 3H9, was described previously.28 Functional inhibition of ADAMTS13 in this model was sufficient to induce severe thrombocytopenia, schistocytic hemolytic anemia, and the appearance of platelet- and VWF-rich thrombi. This preclinical baboon model is therefore relevant to evaluate the in vivo efficacy and safety of ALX-0681 treatment in the context of acquired TTP, and will further support its potential to treat patients suffering from this disorder.
The present study is an evaluation of ALX-0681 in the baboon model of acquired TTP in which the efficacy of ALX-0681 to either prevent or treat acquired TTP was investigated. Safety (bleeding risk) related to ALX-0681 treatment in the context of thrombocytopenia with concomitant neutralization of VWF activity was also monitored in this study by screening for intracranial bleeding and internal organ bleeding.
Methods
Materials
The humanized bivalent ALX-0681 Nanobody consists of 2 identical VWF-binding building blocks (PMP12A2h1) genetically linked to each other with a 3 alanine linker and is produced in Escherichia coli, as described previously.21 The ALX-0681 drug substance formulation buffer (D-PBS, 0.2M glycine, 0.02% Tween 80, pH 7.1 ± 0.1) was used as a vehicle in the study. The TTP-inducing mAb 3H9 was produced and provided by the Katholieke Universiteit Leuven (Flanders, Belgium).28
Animals
Housing, treatment, care and final protocol were approved by the Interfaculty Control Committee for Animal Experimentation of the University of the Free State (Bloemfontein, South Africa) in accordance with South African National Standard for the Care and Use of Animals for Scientific Purpose (SANS 10386).
The animals (wild-caught baboons supplied by Grootfontein Boerdery) were all male, weighed 7.8-14.6 kg (average, 10.4 ± 2.1), and were housed in the holding area on the west campus of the University of the Free State in standard housing cages. There was an acclimatization period of 14 days before study start. Animals were fed dry formulated primate pellets supplied by AquaNutro (formulated by the University of Stellenbosch, Stellenbosch, South Africa) for the duration of the experiment and had free access to water. Animals were weighed once daily and monitored twice daily for signs of TTP or discomfort (ie, fever, lethargy, decreased feeding, bleeding or bruising, and blood in urine) and observations were noted on the animal welfare sheet. No symptoms were observed that warranted euthanasia or treatment interruption in any of the study animals. At the end of the study, animals selected for post mortem analysis and histopathologic evaluation (4 animals of the therapeutic group and 1 control animal) were euthanized by pentobarbitone overdose.
Study design
This study combined a preventive and a therapeutic arm. The overall study outline is indicated in Table 1.
Study design and dosing schedule
| Group . | N . | Doses and administration schedule . | ||
|---|---|---|---|---|
| 3H9, 0.6 mg/kg IV . | Vehicle, SC daily . | ALX-0681, 2.5 mg/kg SC daily . | ||
| Control animals | 4 | d 1, 3, 5, 7, 9 | d 1-11 | |
| Preventive ALX-0681–treated animals | 4 | d 1 and 3 | d 1-5 | |
| Therapeutic ALX-0681–treated animals | 4 | d 1, 3, 5, 7, 9 | d 1-4 | d 5-11 |
| Group . | N . | Doses and administration schedule . | ||
|---|---|---|---|---|
| 3H9, 0.6 mg/kg IV . | Vehicle, SC daily . | ALX-0681, 2.5 mg/kg SC daily . | ||
| Control animals | 4 | d 1, 3, 5, 7, 9 | d 1-11 | |
| Preventive ALX-0681–treated animals | 4 | d 1 and 3 | d 1-5 | |
| Therapeutic ALX-0681–treated animals | 4 | d 1, 3, 5, 7, 9 | d 1-4 | d 5-11 |
Table shows an overview of the timing of injections in the different study groups.
The study consisted of 3 groups (4 baboons per group, 12 baboons in total). Control animals received daily vehicle and 3H9 at 48-hour intervals until day 9 and served as controls for both the preventive and therapeutic study arm. Preventive ALX-0681–treated animals received 3H9 on days 1 and 3 plus daily ALX-0681 from day 1 until day 5. The potential occurrence of TTP symptoms after withdrawal of ALX-0681 treatment was monitored from day 5 to day 11 every 48 hours. Therapeutic ALX-0681–treated animals received 3H9 at 48-hour intervals from day 1 to day 9. During the first 4 days, these animals were also injected daily with vehicle. Induction of TTP was confirmed by platelet count and haptoglobin testing. From day 5 onward, these animals received a daily dose of ALX-0681 for the remaining days.
To yield groups with comparable mean body weights, platelet counts, and VWF levels, study animals were stratified into 1 of the 3 study groups based on prescreening results during the acclimatization period (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The randomization of treatment status for each group was performed by sealed paper drawing. Animals were identified by cage number throughout the study.
Injections and blood sampling
Injections of the compounds (ie, ALX-0681, vehicle, and 3H9) and blood sampling were performed under anesthesia (ketamine-hydrochloride 1 mg/kg of body weight given intramuscularly every 30 minutes). Daily blood sampling was done by venipuncture from the femoral vein using a 21 G Vacutainer blood collection set (BD Biosciences). SC injections were given in the chest area (ALX-0681 or vehicle; approximately 2 minutes before blood sampling) or in the femoral vein (3H9; immediately after blood sampling). The 3H9 mAb was administered by an IV bolus injection of 600 μg/kg in PBS every 48 hours. ALX-0681 was administered by a daily SC injection at a 2.5 mg/kg dose. Vehicle was administered SC using the same dosing volume as ALX-0681. Individual dosing volumes were based on the daily measurement of body weight. Animals were euthanized by pentobarbitone overdose (200 mg/kg of body weight).
Blood analysis
The following blood parameters were determined by automated and standardized methods in the National Health Laboratory Service Tertiary Laboratory (Universitas Hospital, Bloemfontein, South Africa): full blood count, lactate dehydrogenase (LDH), haptoglobin, urea, creatinine, troponin-T, FVIII clotting activity (FVIII:C), and disseminated intravascular coagulation screening. Schistocytes were counted by an expert hematologist. Samples were blinded and counting was done manually.
VWF:Ag and total active ALX-0681 plasma concentrations were determined by validated ELISA assays. Ristocetin cofactor (RICO) activity was determined using a PAP-8E platelet aggregometer (Chrono-log 4 channel aggregometer; Kordia).
Other blood parameters were measured using ELISA kits for human markers: ADAMTS13 antigen (Technozym ADAMTS-13 Antigen ELISA kit, Technoclone), ADAMTS13 activity (ADAMTS13:Act; Technozym ADAMTS-13 Activity ELISA kit, Technoclone), and the brain injury markers neuron-specific enolase (ALPCO Diagnostics) and S100B (DRG International). These kits were validated before sample analysis by analyzing parallelism and by monitoring precision and accuracy of calibrators, validation/quality control samples, and individual baboon samples (data not shown). Although the assays were found to be fit-for-purpose, data should be interpreted semiquantitatively because the baboon plasma levels were determined against a calibrator curve derived from human plasma.
Brain CT scans
All animals of the therapeutic study arm were subjected to a brain CT scan on days 4, 7, and 11 under anesthesia (ketamine-hydrochloride 1 mg/kg of body weight given intramuscularly every 30 minutes or as needed). A CT brain scan was performed on a General Electric HD 750 CT Scanner; 5-mm axial images were taken throughout the brain, including the posterior fossa up to the cortex. These images were interpreted on an Advantage Windows Workstation (General Electric) by a staff radiologist skilled in interpreting brain injury and brain bleeding. If present, intracranial bleeding is represented by high-density areas in the CT of the brain, and bleeding lesions are classified as subdural, extradural, subarachnoid, and intracerebral.
Post mortem analysis
Internal bleeding was evaluated by post mortem analysis with macroscopic examination and histopathologic assessment of selected organs (major parenchymas). Only the animals of the therapeutic group and 1 control animal were evaluated. Histopathologic evaluation of aggregates was included for these animals, as described previously.28 Briefly, wedges of lung, heart, brain, kidney, and spleen were dissected and fixed in 10% buffered formaldehyde for 24 hours. A Tissue Tek microtome/cryostat device (Bayer Healthcare) was used for processing and embedding. H&E staining was performed by standard techniques. Staining for VWF and platelets was done with a polyclonal anti-VWF (P0226) and the mAb anti–human glycoprotein IIIa clone Y2/51 (M0753), respectively, both peroxidase labeled (Dako Denmark). 3-3-Diaminobenzidine was used as a chromogenic substrate.
Statistical analysis
Separate models were constructed for the first period (days 1-5) and the second period (days 6-11). Based on the pharmacokinetic (PK) profile of ALX-0681 in the animals, it was chosen to perform comparisons between groups when ALX-0681 was at steady state. Therefore, in the first period, the comparison was done on days 3, 4, and 5 and in the second period on days 9, 10, and 11. All analyses were performed in SAS Version 9.2 software (SAS Institute).
In the first period, the control animals (n = 4) and the therapeutically treated animals (n = 4) were pooled in 1 group for statistical analysis, because these animals were treated equally in that period. Consequently, the preventively treated group was compared with this pooled group during the first period. In the second period, comparisons were done compared with the control group.
The variable RICO was converted to categorical because it was either considered to be completely suppressed (RICO ≤ 20%) or not. The RICO results were analyzed with a χ2 test for the first and second period separately. Troponin-T was analyzed by nonparametric statistics because the residuals were not distributed normally. In the first period, the Wilcoxon test was used and in the second period the Kruskal-Wallis test. All other variables were analyzed with a mixed model having a random intercept per animal.29 For the fixed effects in the mixed model, the model selection started off from the model that includes day, treatment, and day*treatment. Because in the mixed models a profile analysis without assumptions on the shape of the evolution over time was performed,29 day was entered in the model as a categorical variable. When not significant, the effect day*treatment was removed. A posthoc test with Tukey multiple comparison adjustment30 assessed which groups specifically differed and at which point in time. Log transformation of the response was applied when needed to achieve normal distribution.
Results
3H9 completely neutralizes ADAMTS13:Act
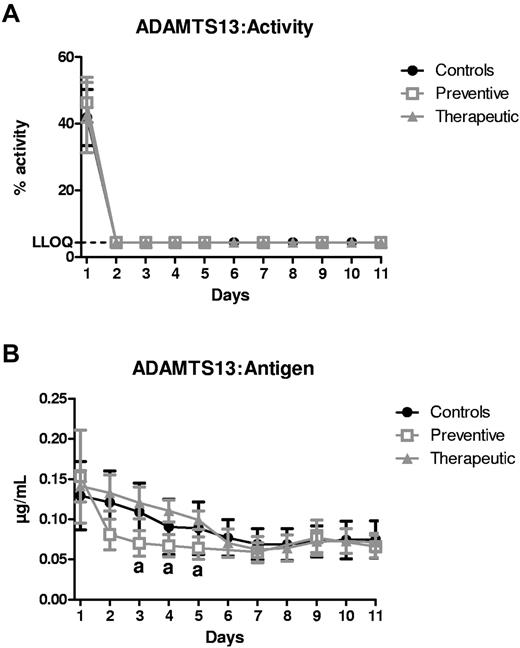
Injection of 3H9 every 48 hours resulted in a rapid and sustained suppression (below the lower limit of quantification of 4.4%) of ADAMTS13:Act in all animals (Figure 1A). Remarkably, in the animals of the prevention study group that received 3H9 only twice (days 1 and 3), ADAMTS13:Act remained inhibited for the entire study period, suggestive of a long half-life of the mAb. Concomitantly, ADAMTS13:Antigen levels moderately decreased in all study animals (Figure 1B), indicating partial clearance by injection of 3H9.
Inhibition of ADAMTS13 parameters by 3H9. (A) ADAMTS13:Activity (%) and (B) ADAMTS13:Antigen (μg/mL) were measured in plasma of the control (●; n = 4), preventive (□; n = 4), and therapeutic ( ; n = 4) animals as a function of time (days). The lower limit of quantification of the ADAMTS13:Activity assay was determined to be 4.4% and is indicated by the dashed line. Results were analyzed with a mixed model and a posthoc test with Tukey multiple comparison adjustment. Data are represented as means ± SD. “a” indicates a significant difference between control and preventive animals (P < .05).
; n = 4) animals as a function of time (days). The lower limit of quantification of the ADAMTS13:Activity assay was determined to be 4.4% and is indicated by the dashed line. Results were analyzed with a mixed model and a posthoc test with Tukey multiple comparison adjustment. Data are represented as means ± SD. “a” indicates a significant difference between control and preventive animals (P < .05).
Inhibition of ADAMTS13 parameters by 3H9. (A) ADAMTS13:Activity (%) and (B) ADAMTS13:Antigen (μg/mL) were measured in plasma of the control (●; n = 4), preventive (□; n = 4), and therapeutic ( ; n = 4) animals as a function of time (days). The lower limit of quantification of the ADAMTS13:Activity assay was determined to be 4.4% and is indicated by the dashed line. Results were analyzed with a mixed model and a posthoc test with Tukey multiple comparison adjustment. Data are represented as means ± SD. “a” indicates a significant difference between control and preventive animals (P < .05).
; n = 4) animals as a function of time (days). The lower limit of quantification of the ADAMTS13:Activity assay was determined to be 4.4% and is indicated by the dashed line. Results were analyzed with a mixed model and a posthoc test with Tukey multiple comparison adjustment. Data are represented as means ± SD. “a” indicates a significant difference between control and preventive animals (P < .05).
ALX-0681 prevents and treats the acute early episodes of acquired TTP in baboons
Inhibition of ADAMTS13:Act resulted in a rapid onset and pronounced development of thrombocytopenia and schistocytic hemolytic anemia in the control animals (Figure 2A-D). These major hallmarks of acquired TTP were confirmed by a fast and persistent suppression of platelet count and haptoglobin levels (a marker of intravascular hemolysis), and increases in RBC fragmentation (schistocytes) and LDH levels (marker of tissue damage and hemolysis; Figure 2A-D). In addition, RBC counts and hemoglobin concentrations were decreased as a result of TTP induction by 3H9 (supplemental Figure 1). There were no signs of kidney failure (creatinine and urea) or brain injury (neuron-specific enolase or S100B) in any of the study groups (supplemental Figure 2). There was 1 control animal with pronounced increases in troponin-T levels suggestive of myocardial infarction. This finding was confirmed by post mortem analysis showing signs of myocardial damage (tissue necrosis) in this animal.
Preventive and therapeutic effect of ALX-0681 on the markers of thrombocytopenia and schistocytic hemolytic anemia. Platelet counts (109/L; A), haptoglobin levels (g/L; B), schistocyte counts (%; C), and LDH levels (U/L; D) were measured in control (●; n = 4), preventive (□; n = 4), and therapeutic ( ; n = 4) animals as a function of time (days). Results were analyzed with a mixed model and a posthoc test with Tukey multiple comparison adjustment. Data are represented as means ± SD. “a” indicates a significant difference between control and preventive animals (P < .05); “b,” significant difference between control and preventive animals (P < .001); “c,” significant difference between control and therapeutic animals (P < .05); and “d,” significant difference between control and therapeutic animals (P < .001).
; n = 4) animals as a function of time (days). Results were analyzed with a mixed model and a posthoc test with Tukey multiple comparison adjustment. Data are represented as means ± SD. “a” indicates a significant difference between control and preventive animals (P < .05); “b,” significant difference between control and preventive animals (P < .001); “c,” significant difference between control and therapeutic animals (P < .05); and “d,” significant difference between control and therapeutic animals (P < .001).
Preventive and therapeutic effect of ALX-0681 on the markers of thrombocytopenia and schistocytic hemolytic anemia. Platelet counts (109/L; A), haptoglobin levels (g/L; B), schistocyte counts (%; C), and LDH levels (U/L; D) were measured in control (●; n = 4), preventive (□; n = 4), and therapeutic ( ; n = 4) animals as a function of time (days). Results were analyzed with a mixed model and a posthoc test with Tukey multiple comparison adjustment. Data are represented as means ± SD. “a” indicates a significant difference between control and preventive animals (P < .05); “b,” significant difference between control and preventive animals (P < .001); “c,” significant difference between control and therapeutic animals (P < .05); and “d,” significant difference between control and therapeutic animals (P < .001).
; n = 4) animals as a function of time (days). Results were analyzed with a mixed model and a posthoc test with Tukey multiple comparison adjustment. Data are represented as means ± SD. “a” indicates a significant difference between control and preventive animals (P < .05); “b,” significant difference between control and preventive animals (P < .001); “c,” significant difference between control and therapeutic animals (P < .05); and “d,” significant difference between control and therapeutic animals (P < .001).
Prophylactic administration of ALX-0681 not only prevented the rapid onset of thrombocytopenia after 3H9 injection, but schistocytosis, intravascular hemolysis, and increases in LDH levels were completely and significantly prevented as well (Figure 2A-D). The decrease in RBC counts and hemoglobin concentrations was avoided in the preventive study group (significant difference compared with control animals on day 9 and day 11; supplemental Figure 1). In parallel with the sustained suppression of ADAMTS13:Act in these animals, a decrease in platelet count could still be observed after treatment cessation (Figure 2A).
In the therapeutic arm of the study, induction of TTP was first confirmed on day 5 before initiating ALX-0681 treatment. A similar decrease in platelet count and haptoglobin levels and comparable increases in schistocyte count and LDH levels were noted in the animals of the therapeutic study group compared with control animals until day 5 (Figure 2A-D). Daily injections with ALX-0681 were started on day 5 and were followed by an immediate and steep increase of the platelet count. Predose platelet counts were reached on day 9, which was followed by an overshoot on day 11 (+80% mean increase on day 11 compared with day 1 counts). In addition, the induction of the schistocytic hemolytic anemia could be effectively treated with ALX-0681, as shown in Figure 2B through D. Further increases in schistocyte count after day 5 were entirely blocked. A trend toward normalization of RBC fragmentation could be noted, supported by significant differences between control and treated animals on days 9, 10, and 11 (Figure 2C). Similarly, haptoglobin concentrations started to normalize in the therapeutic study group from day 10, together with significantly lower LDH concentrations from day 9 compared with control animals (Figure 2B and D). Hemoglobin and RBC count were similarly decreased in the therapeutic animals compared with the control animals (supplemental Figure 1). It is likely that the follow-up period was too short to observe a reversal of these parameters because haptoglobin concentrations started to recover only from day 10.
Immunohistochemical scoring of occluded vessels in the kidneys, heart, spleen, brain, and lungs of the animals of the therapeutic study group and 1 control animal confirmed the presence of VWF- and platelet-rich thrombi. A similar occlusion score was observed in treated animals compared with the control animal (Table 2). Occlusions were most prominently observed in the brain and were also seen in the heart, kidneys, and spleen, but not in the lungs. These data suggest that ALX-0681 treatment was not able to dissolve already formed thrombi in the vessels, but rather prevented the progressing formation of aggregates, as would be expected from the preventive mode of action. In fact, in vitro flow chamber data showed that ALX-0681 completely blocked ULVWF-mediated platelet string formation when added to the platelet suspension before perfusion over stimulated endothelial cells (supplemental Methods and supplemental Figure 3). However, ALX-0681 was not able to detach these platelets if they were allowed to form platelet strings before ALX-0681 addition (supplemental Figure 3).
Occlusion scores for all organs
| Organ . | Animal . | |||||
|---|---|---|---|---|---|---|
| C4 . | C5 . | C6 . | C7 . | Mean (C4-C7) . | D10 . | |
| CD61-rich occlusion scores, % | ||||||
| Kidney | 0 | 0 | 0 | 10 | 2.5 | 0 |
| Spleen | 0 | 0 | 0 | 0 | 0 | 0 |
| Heart | 10 | 10 | 10 | 0 | 7.5 | 10 |
| Lung | 0 | 0 | 0 | 0 | 0 | 0 |
| Brain | 20 | 40 | 50 | 40 | 37.5 | 40 |
| VWF-rich occlusion scores, % | ||||||
| Kidney | 0 | 0 | 0 | 10 | 2.5 | 0 |
| Spleen | 0 | 0 | 0 | 10 | 2.5 | 0 |
| Heart | 20 | 20 | 0 | 0 | 10 | 20 |
| Lung | 0 | 0 | 0 | 0 | 0 | 0 |
| Brain | 20 | 30 | 60 | 50 | 40 | 20 |
| Organ . | Animal . | |||||
|---|---|---|---|---|---|---|
| C4 . | C5 . | C6 . | C7 . | Mean (C4-C7) . | D10 . | |
| CD61-rich occlusion scores, % | ||||||
| Kidney | 0 | 0 | 0 | 10 | 2.5 | 0 |
| Spleen | 0 | 0 | 0 | 0 | 0 | 0 |
| Heart | 10 | 10 | 10 | 0 | 7.5 | 10 |
| Lung | 0 | 0 | 0 | 0 | 0 | 0 |
| Brain | 20 | 40 | 50 | 40 | 37.5 | 40 |
| VWF-rich occlusion scores, % | ||||||
| Kidney | 0 | 0 | 0 | 10 | 2.5 | 0 |
| Spleen | 0 | 0 | 0 | 10 | 2.5 | 0 |
| Heart | 20 | 20 | 0 | 0 | 10 | 20 |
| Lung | 0 | 0 | 0 | 0 | 0 | 0 |
| Brain | 20 | 30 | 60 | 50 | 40 | 20 |
Organs were examined for occluded vessels rich in platelets (monoclonal mouse anti–human CD61) or VWF (polyclonal rabbit anti–human VWF). Ten random consecutive vessels were scored for occlusions in animals from the therapeutic study group (C4-C7) and 1 control animal (D10). Results are represented as the percentage of positive vessels. Mean (C4-C7) represents the average occlusion (%) per organ of the 4 animals in the therapeutic study group.
Pharmacodynamic markers and PK profile of ALX-0681
The prevention or reversal of the clinical signs of acquired TTP were correlated fully with suppression of the pharmacodynamic marker RICO, as shown in Figure 3. RICO activity was suppressed (below the pharmacologic threshold of 20%) in both treatment groups the day after the first administration of ALX-0681. In addition, the pharmacodynamic marker recovered above the threshold level after treatment cessation from day 7 onward in the preventive study group. The latter is consistent with the observed ALX-0681 plasma concentration-time profiles (Table 3).
ALX-0681 neutralizes VWF activity as shown by suppression of RICO. RICO activity was measured in plasma of the control (●; n = 4), preventive (□; n = 4), and therapeutic ( ; n = 4) animals as a function of time (days). The lower limit of quantification (LLOQ) and upper limit of quantification (ULOQ) of the RICO assay were 10% and 145%, respectively, and are indicated by a dashed line. The pharmacologic threshold is established at 20% activity and is indicated by a dotted line. Results were analyzed with a χ2 test. Data are represented as means ± SD. “a” indicates a significant difference between control and preventive animals (P < .01); “b,” a significant difference between control and therapeutic animals (P < .01).
; n = 4) animals as a function of time (days). The lower limit of quantification (LLOQ) and upper limit of quantification (ULOQ) of the RICO assay were 10% and 145%, respectively, and are indicated by a dashed line. The pharmacologic threshold is established at 20% activity and is indicated by a dotted line. Results were analyzed with a χ2 test. Data are represented as means ± SD. “a” indicates a significant difference between control and preventive animals (P < .01); “b,” a significant difference between control and therapeutic animals (P < .01).
ALX-0681 neutralizes VWF activity as shown by suppression of RICO. RICO activity was measured in plasma of the control (●; n = 4), preventive (□; n = 4), and therapeutic ( ; n = 4) animals as a function of time (days). The lower limit of quantification (LLOQ) and upper limit of quantification (ULOQ) of the RICO assay were 10% and 145%, respectively, and are indicated by a dashed line. The pharmacologic threshold is established at 20% activity and is indicated by a dotted line. Results were analyzed with a χ2 test. Data are represented as means ± SD. “a” indicates a significant difference between control and preventive animals (P < .01); “b,” a significant difference between control and therapeutic animals (P < .01).
; n = 4) animals as a function of time (days). The lower limit of quantification (LLOQ) and upper limit of quantification (ULOQ) of the RICO assay were 10% and 145%, respectively, and are indicated by a dashed line. The pharmacologic threshold is established at 20% activity and is indicated by a dotted line. Results were analyzed with a χ2 test. Data are represented as means ± SD. “a” indicates a significant difference between control and preventive animals (P < .01); “b,” a significant difference between control and therapeutic animals (P < .01).
Plasma concentrations of ALX-0681 in animals of the preventive (D3-D6) and therapeutic (C4-C7) study groups
| d . | Calculated plasma concentration of ALX-0681, ng/mL . | |||||||
|---|---|---|---|---|---|---|---|---|
| Animal . | ||||||||
| D3 . | D4 . | D5 . | D6 . | C4 . | C5 . | C6 . | C7 . | |
| 1 | 32.46 | BQL | BQL | BQL | BQL | ND | ND | ND |
| 2 | 170.97 | 150.03 | 308.16 | 231.94 | BQL | ND | ND | ND |
| 3 | 161.14 | 134.31 | 263.78 | 180.57 | BQL | ND | ND | ND |
| 4 | 169.69 | 135.66 | 189.68 | 170.01 | BQL | ND | ND | ND |
| 5 | 165.41 | 156.76 | 222.44 | 188.99 | BQL | ND | ND | ND |
| 6 | ND | ND | ND | ND | 407.37 | 257.15 | 271.84 | 246.37 |
| 7 | 81.45 | 82.06 | 129.64 | 99.05 | 310.89 | 155.87 | 204.31 | 176.11 |
| 8 | ND | ND | ND | ND | 324.30 | 179.62 | 266.17 | 209.12 |
| 9 | 26.23 | BQL | 55.06 | 47.57 | 253.21 | 172.01 | 230.45 | 209.25 |
| 10 | ND | ND | ND | ND | 258.37 | 145.34 | 235.66 | 231.66 |
| 11 | BQL | BQL | BQL | BQL | 286.81 | 151.96 | 210.20 | 201.96 |
| d . | Calculated plasma concentration of ALX-0681, ng/mL . | |||||||
|---|---|---|---|---|---|---|---|---|
| Animal . | ||||||||
| D3 . | D4 . | D5 . | D6 . | C4 . | C5 . | C6 . | C7 . | |
| 1 | 32.46 | BQL | BQL | BQL | BQL | ND | ND | ND |
| 2 | 170.97 | 150.03 | 308.16 | 231.94 | BQL | ND | ND | ND |
| 3 | 161.14 | 134.31 | 263.78 | 180.57 | BQL | ND | ND | ND |
| 4 | 169.69 | 135.66 | 189.68 | 170.01 | BQL | ND | ND | ND |
| 5 | 165.41 | 156.76 | 222.44 | 188.99 | BQL | ND | ND | ND |
| 6 | ND | ND | ND | ND | 407.37 | 257.15 | 271.84 | 246.37 |
| 7 | 81.45 | 82.06 | 129.64 | 99.05 | 310.89 | 155.87 | 204.31 | 176.11 |
| 8 | ND | ND | ND | ND | 324.30 | 179.62 | 266.17 | 209.12 |
| 9 | 26.23 | BQL | 55.06 | 47.57 | 253.21 | 172.01 | 230.45 | 209.25 |
| 10 | ND | ND | ND | ND | 258.37 | 145.34 | 235.66 | 231.66 |
| 11 | BQL | BQL | BQL | BQL | 286.81 | 151.96 | 210.20 | 201.96 |
BQL indicates below the quantification limit of 20 ng/mL; and ND, not determined.
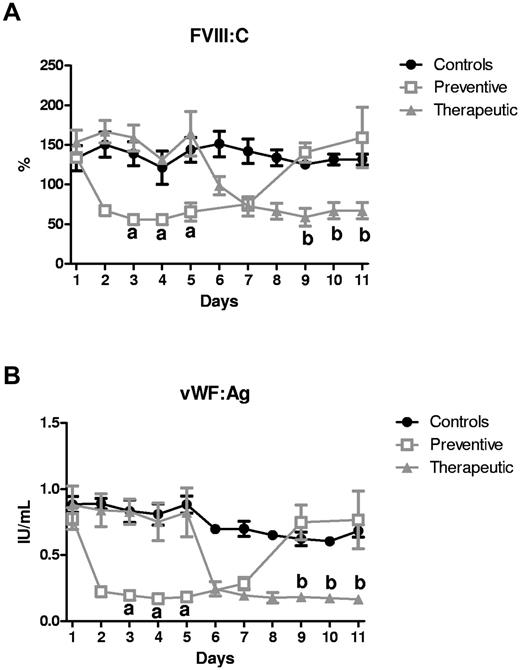
There was a significant decrease in FVIII:C and VWF:Ag levels on repeated administration of ALX-0681 in both the preventive and therapeutic study groups (Figure 4A-B). The levels quickly returned to predose levels after stopping ALX-0681 treatment. The changes in FVIII:C and VWF:Ag concentration were manageable in terms of coagulation, because other markers for coagulation (prothrombin time, activated partial thromboplastin time, and thrombin time) were all within normal ranges (supplemental Table 2).
Repeated ALX-0681 administration reversibly decreases the FVIII:C and VWF:Ag concentrations. FVIII:C (%; A) and VWF:Ag (B) were measured in baboon plasma of the control (●; n = 4), preventive (□; n = 4), and therapeutic ( ; n = 4) animals as a function of time (days). Results were analyzed with a mixed model and a posthoc test with Tukey multiple comparison adjustment. Data are represented as means ± SD. “a” indicates a significant difference between control and preventive animals (P < .001); “b,” a significant difference between control and therapeutic animals (P < .001).
; n = 4) animals as a function of time (days). Results were analyzed with a mixed model and a posthoc test with Tukey multiple comparison adjustment. Data are represented as means ± SD. “a” indicates a significant difference between control and preventive animals (P < .001); “b,” a significant difference between control and therapeutic animals (P < .001).
Repeated ALX-0681 administration reversibly decreases the FVIII:C and VWF:Ag concentrations. FVIII:C (%; A) and VWF:Ag (B) were measured in baboon plasma of the control (●; n = 4), preventive (□; n = 4), and therapeutic ( ; n = 4) animals as a function of time (days). Results were analyzed with a mixed model and a posthoc test with Tukey multiple comparison adjustment. Data are represented as means ± SD. “a” indicates a significant difference between control and preventive animals (P < .001); “b,” a significant difference between control and therapeutic animals (P < .001).
; n = 4) animals as a function of time (days). Results were analyzed with a mixed model and a posthoc test with Tukey multiple comparison adjustment. Data are represented as means ± SD. “a” indicates a significant difference between control and preventive animals (P < .001); “b,” a significant difference between control and therapeutic animals (P < .001).
ALX-0681 treatment is not linked with bleeding events
Bleeding related to ALX-0681 treatment of acquired TTP in the context of thrombocytopenia and neutralization of VWF activity was monitored in the therapeutic study group. There was no indication of relevant bleeding events during the in-life phase, apart from the expected bruising at injection sites and mild transient gingival bleeding. Animals were also screened for intracranial bleeding and checked for internal organ bleeding through brain CT scans and post mortem analysis of the organs, respectively. No signs of intracranial bleeding were seen in any of the scans of the 4 ALX-0681–treated animals. Moreover, the absence of any intracranial bleeding was further confirmed by the absence of any macroscopic and/or histologic observation of bleeding in any of the organs of the animals examined.
Discussion
The formation of platelet-rich thrombi in the microvasculature is a key characteristic of TTP patients because of an acquired deficiency of ADAMTS13.31,32 In the absence of ADAMTS13, the processing of ULVWF multimers is impaired and leads to the spontaneous interaction of VWF and platelets. This enhanced platelet aggregation can cause severe organ damage and long-term neurologic disorders and is linked with pronounced morbidity and mortality.17 Given the crucial role of this abnormal interaction of VWF with platelets in the pathophysiology of TTP, targeting the VWF A1 domain and preventing the formation of these thrombi offers an attractive new therapeutic concept for the treatment of acquired TTP.
A preclinical baboon model that mimics the acute early episodes of acquired TTP has been described previously.28 This model provided evidence for a direct relationship between the induction of TTP and ADAMTS13 deficiency, leading to the inability to process ULVWF and the formation of platelet-rich aggregates responsible for the clinical symptoms of acquired TTP.33,34 Consistent with this earlier report, the results of the present study show that administration of the ADAMTS13-inhibiting mAb 3H9 resulted in a similar rapid onset of early acute episodes of acquired TTP. The pathophysiology of TTP in this preclinical model seems to differ to some extent from the human situation, because ADAMTS13 deficiency alone suffices to induce TTP in these animals. Additional exogenous triggers are hypothesized to accelerate and aggravate disease progression in humans, possibly leading to a more refractory disease, even though the exact triggers are not always known.35-37 Moreover, other typical clinical manifestations of TTP, such as neurologic symptoms and renal failure, have not been reported in this model, suggesting a mild expression of the disease.28 However, it has been suggested that the initial symptoms of TTP patients at presentation may be variable as well, and that not all patients present with neurologic symptoms or renal failure.38 Therefore, this preclinical baboon model is a very suitable model with which to test the potential of new treatment opportunities for acquired TTP.
The antithrombotic Nanobody has been shown previously to inhibit the interaction between normal-sized VWF multimers and platelets in nonclinical and clinical studies.21,39,40 Based on its mechanism of action, it is hypothesized that ALX-0681 is also able to prevent the ULVWF-mediated formation of platelet-rich microthrombi. The efficacy of ALX-0681 in targeting ULVWF and spontaneous platelet aggregation was therefore investigated herein in a baboon model of acquired TTP. The prophylactic effect of ALX-0681 on the induction of thrombocytopenia and schistocytic hemolytic anemia seen in the present study strongly supports the concept that ULVWF is a promising target to mediate the pathophysiology of TTP. The tendency toward thrombocytopenia and hemolytic anemia after cessation of ALX-0681 treatment further confirms the target-specific effect of ALX-0681 because the disease-inducing mAb 3H9 is expected to remain in the circulation for a longer time. In the clinical situation, treatment duration for ALX-0681 therapy is tailored with the disposition of anti-ADAMTS13 inhibitors for plasma exchange.
The introduction of plasma exchange has reduced the mortality rates for TTP patients dramatically. However, the condition still carries a significant risk of mortality (10%-30%) and morbidity, which is related to the high risk of complications linked to the procedure.16,17,19,41 Given this sustained level of mortality in the treatment of TTP, ALX-0681 offers a novel approach toward treating the disease and can reduce the need for plasma exchange. In fact, plasma exchange removes the autoantibodies against ADAMTS13 as well as the ULVWF multimers while simultaneously supplying ADAMTS13, but does not provide direct pharmacologic targeting of the pathophysiology of TTP: ULVWF-mediated platelet aggregation. A clinical study with the anti-VWF aptamer ARC1779, which also neutralizes the VWF A1 domain, has provided a preliminary proof-of-concept of the novel approach to targeting the A1 domain in the treatment of TTP.25 So far, only limited data are available showing clinically significant improvements of platelet counts and LDH levels in some but not all TTP patients treated with ARC1779.24 Given the higher potency and superior PK properties of ALX-0681 over ARC1779, it is expected that ALX-0681 shows a more favorable effect toward inhibition of ULVWF-mediated platelet aggregation. The data reported in this study indicate that treatment with the anti-VWF Nanobody rapidly reversed the pronounced drop in platelet count, reaching baseline levels after 4 days of treatment. In addition, the schistocytic hemolytic anemia was immediately stopped upon initiation of ALX-0681 treatment together with a significant normalization of LDH levels, which were perfectly correlated with full neutralization of VWF activity. Therefore, these data indicate that ALX-0681 has the potential to inhibit the progressing formation of platelet-rich thrombi in the microvasculature. In addition, its marked effect on LDH and haptoglobin levels highlights its potential benefits of reducing ischemic and hemorrhagic complications.
In the clinical setting, early onset of treatment with ALX-0681 and sufficient treatment duration needs to be considered. The anti-VWF Nanobody does not eliminate the anti-ADAMTS13 Abs as the root cause of idiopathic TTP, nor is it expected to dissolve platelet-rich ULVWF aggregates based on the vessel occlusion data and the in vitro perfusion data. However, it is anticipated that ALX-0681 may significantly reduce the time to normalization of platelet counts and the number of plasma-exchange sessions needed to achieve remission, and therefore may subsequently reduce the risk of complications linked to the plasma-exchange procedure (eg, systemic infections, catheter obstruction or insertion complications, hypotension, and venous thrombosis).17 The inhibition of microvascular thrombosis is expected to reduce organ dysfunction, accelerate organ recovery, and improve long-term neurologic disorders in TTP.42 It may also provide an interesting alternative for immunosuppressive treatment, which is traditionally initiated for refractory or relapsing patients.18 These therapeutic benefits of ALX-0681 in addition to plasma exchange are expected to have a significant impact on the quality of life of TTP patients.
The present study investigated not only the efficacy, but also the safety of ALX-0681 treatment for the acute onset of TTP. The most relevant safety concern of the anti-VWF compound ALX-0681 in the treatment of TTP is an elevated bleeding risk in the context of low platelet counts and reduced VWF activity. Although this study was not adequately powered to fully address the safety profile of ALX-0681, the current data showed no bleeding events in any of the animals of the therapeutic group, which would exclude a severe bleeding risk of this compound. These observations are further confirmed by a lower bleeding potential of ALX-0681 in a preclinical surgical bleeding model compared with other marketed antithrombotics21 and by the absence of spontaneous significant bleeding in phase 1 trials performed with ALX-0681.39,40 Repeated administration of ALX-0681 also resulted in significant decreases of VWF:Ag and FVIII:C concentrations, which were rapidly normalized on treatment cessation. Mild and transient reductions in FVIII and VWF were also observed in single or multiple dosing phase 1 trials with the antithrombotic Nanobody.39,40 These changes can be attributed to the pharmacology of ALX-0681.21 PK simulation has pointed toward a change in the elimination rate of the ALX-0681-VWF-FVIII complex compared with the clearance of VWF alone.21 The observed reductions in VWF and FVIII correspond to the range of VWF levels in patients with type 1 or type 2 VWD.43 These patients typically have 15%-50% of normal VWF plasma levels with proportional decreases in FVIII concentrations,44 and generally present with mild bleeding symptoms. TTP patients present with elevated VWF serum concentrations, and a reduction to normal levels can support the beneficial effect of ALX-0681. Overall, these data suggest that ALX-0681 treatment has a manageable safety profile in terms of clinical bleeding risk, even under conditions of thrombocytopenia and full neutralization of VWF activity.
In conclusion, this study in a preclinical baboon model of acquired TTP convincingly demonstrated that ALX-0681 can prevent and treat the acute early episodes of acquired TTP by targeting the VWF-platelet interaction. Full neutralization of VWF activity by ALX-0681 resulted in efficacious treatment of the most important hallmarks of TTP: severe thrombocytopenia and schistocytic hemolytic anemia. Moreover, no signs of an excessive bleeding risk related to ALX-0681 treatment were observed. These preclinical data further strengthen the promising novel concept of inhibiting ULVWF-mediated platelet aggregation as an add-on treatment for TTP patients and fully support the ongoing phase 2 clinical trial assessing the efficacy and safety of ALX-0681 as an adjunctive treatment for patients with acquired TTP.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Jasper Jacobs, Sarah De Pauw, and Kris Cosyns for technical support during sample analysis; Antoine Thomas for supplying and characterizing ALX-0681 and formulation buffer; Dr Elson Mberi for counting schistocytes; Prof Curt De Vries for performing and interpreting the brain CT scans; and Dr Ton Lisman (Department of Hematology of the University Medical Center, Utrecht, The Netherlands) for performing the flow chamber experiments.
The study was paid for by Ablynx NV. Nanobody is a registered trademark of Ablynx NV.
Authorship
Contribution: F.C. designed and performed the research, analyzed and interpreted the data, and wrote the manuscript; J.R. designed and performed the research, analyzed and interpreted the data, and critically read the manuscript; H.U., T.S., S.R., S.P., and J.-B.H. designed the research and analyzed and interpreted the data; W.J.v.R. and S.L. performed the research; and W.W. analyzed the data and performed the statistical analysis.
Conflict-of-interest disclosure: F.C., H.U., T.S., S.R., S.P., W.W., and J.-B.H. are employees of and own shares and/or stock options with Ablynx NV. The remaining authors declare no competing financial interests.
Correspondence: Filip Callewaert, Technologiepark 21, 9052 Zwijnaarde, Belgium; e-mail: filip.callewaert@ablynx.com.
References
Author notes
F.C. and J.R. contributed equally to this work.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal