Abstract
Sickle cell disease is a common hemolytic disorder with a broad range of complications, including vaso-occlusive episodes, acute chest syndrome (ACS), pain, and stroke. Heme oxygenase-1 (gene HMOX1; protein HO-1) is the inducible, rate-limiting enzyme in the catabolism of heme and might attenuate the severity of outcomes from vaso-occlusive and hemolytic crises. A (GT)n dinucleotide repeat located in the promoter region of the HMOX1 gene is highly polymorphic, with long repeat lengths linked to decreased activity and inducibility. We examined this polymorphism to test the hypothesis that short alleles are associated with a decreased risk of adverse outcomes (hospitalization for pain or ACS) among a cohort of 942 children with sickle cell disease. Allele lengths varied from 13 to 45 repeats and showed a trimodal distribution. Compared with children with longer allele lengths, children with 2 shorter alleles (4%; ≤ 25 repeats) had lower rates of hospitalization for ACS (incidence rate ratio 0.28, 95% confidence interval, 0.10-0.81), after adjusting for sex, age, asthma, percentage of fetal hemoglobin, and α-globin gene deletion. No relationship was identified between allele lengths and pain rate. We provide evidence that genetic variation in HMOX1 is associated with decreased rates of hospitalization for ACS, but not pain. This study is registered at www.clinicaltrials.gov as #NCT00072761.
Introduction
Sickle cell disease (SCD) is a common hemolytic disorder resulting in a chronic inflammatory state.1,2 Although this disease is caused by a single base pair mutation in the hemoglobin β-chain gene, persons with SCD can experience varying severity of a number of complications, including vaso-occlusive episodes that can result in acute chest syndrome (ACS), pain, and stroke. Sickle erythrocytes have high rates of lysis, releasing large amounts of hemoglobin into the plasma. Free heme released from hemoglobin induces vascular inflammation and is a major source of oxidative stress among persons with SCD.3
Heme oxygenase is the rate-limiting enzyme responsible for the catabolism of heme. The inducible isoform, heme oxygenase-1, is found expressed in various tissues, including those of the liver, spleen, lung, brain, and endothelium, and is up-regulated by stimuli, such as heme, oxidants, hypoxia, and certain cytokines.4 The cytoprotective effect of HO-1 can be mediated through the anti-inflammatory and antioxidant effects of the degradation of heme, as well as the action of some of its products of catabolism.5,6
HO-1 activity has been implicated as an ameliorator of vaso-occlusion in SCD. Belcher et al7 found that increased and attenuated expression levels of HO-1 were associated with modulation of vascular inflammation with decreased and increased red blood stasis, respectively, in transgenic SCD mice. A number of studies have examined DNA polymorphisms in the HMOX1 gene that might influence the level of heme oxygenase response.8 To date, 2 promoter variants (a −413A > T single-base change and a (GT)n microsatellite polymorphism) have been suggested to have functional roles9,10 in modulating heme oxygenase levels. The (GT)n repeat is highly polymorphic, and accumulating evidence has suggested that persons with lower numbers of repeats might have higher inducible heme oxygenase expression.11,12
Given that inducible HMOX1 gene activity is associated with promoter polymorphisms and that decreased HO-1 activity has been implicated in vascular stasis in transgenic SCD mice, we postulated that genetic variation in HMOX1 would affect the rate of vaso-occlusive events in children with SCD. In this study, we tested whether alleles associated with increased HO-1 activity, compared with alleles associated with decreased HO-1 activity, would be associated with decreased rates of hospitalization for vaso-occlusive pain or ACS episodes and provide evidence for a role of the heme oxygenase system in the etiology of vaso-occlusive crises in children with SCD.
Methods
Study population
The design and patient recruitment for the Silent Cerebral Infarct Transfusion (SIT) Trial is described elsewhere.13 Each clinical center obtained institutional review board approval of the protocol, consent form, and certification for the study procedure. The study was conducted in accordance with the Declaration of Helsinki. Briefly, children 5-14 years of age with SCD (hemoglobin SS) or sickle βO thalassemia (hemoglobin SβO) were enrolled at 29 clinical sites across North America and Europe from 2004 through 2010. To be enrolled, persons had to have an established relationship with the hematologist at the local site. Patients on regular transfusion therapy or receiving hydroxyurea therapy were excluded from the study.
Clinical definitions and data collection
Pain and ACS events were recorded retrospectively at enrollment; therefore, participants were not on blood transfusion therapy when these data were collected. All vaso-occlusive pain episodes and ACS episodes that required hospitalization over a 3-year period before enrollment were recorded for each patient.13,14
A rigorous definition of pain (ie, pain requiring hospitalization) was used to reduce the potential subjectivity associated with assessment of pain in children. Pain events were defined locally as episodes that could not be attributed to causes other than SCD and required hospitalization and treatment with opiates. Local site coordinators verified these episodes for the participants in the highest 10% for rates of painful events.
ACS was defined locally, based on a commonly accepted constellation of criteria that included fever, increased respiratory rate, decreased oxygen saturation, a new radiodensity of chest radiograph, and pneumonia.15-17 If the ACS event occurred during hospitalization for a vaso-occlusive crisis, it was classified as an ACS. The prevalence of ACS across the sites varied within the range of expected rates, and local site coordinators verified events for the participants in the highest 10% for rates of ACS.
Children with asthma were identified by a positive response from a parent or legal guardian to the question from The Third National Health and Nutritional Examination Survey “Has a doctor ever told you that your child has asthma?”18 This strategy for ascertainment of a lifetime diagnosis has been used by the Centers for Disease Control and Prevention to assess prevalence of asthma and in multiple clinical research studies.19-21
DNA analysis
DNA for this study was isolated from EBV-transformed lymphoblast cells established from lymphocytes isolated from the blood of participants enrolled in the SIT Trial. Genotyping of the HMOX1 −413A > T promoter single nucleotide polymorphism (SNP; rs2071746) was performed with a TaqMan SNP Genotyping Assay according to the manufacturer's protocol (Applied Biosystems). After PCR amplification, fluorescence signals from samples and no-template controls were analyzed on a 7900HT Fast Real-Time PCR System (Applied Biosystems). Genotypes were determined by manually reviewing each allelic discrimination plot with Sequence Detection System Version 2.3 software (Applied Biosystems). Each assay plate had a ≥ 95% call rate, and multiple samples of each of the 3 possible genotypes were repeated, with 100% concordant results.
HMOX1 promoter (GT)n dinucleotide repeat (rs3074372) length was determined by PCR fragment size analysis. The 5′ flanking region of the gene was amplified by PCR using an FAM-labeled forward primer (AGA GCC TGC AGC TTC TCA GA) and an unlabeled reverse primer (ACA AAG TCT GGC CAT AGG AC). After amplification, samples were run with an internal size standard (GeneScan-500 LIZ) in Hi-Di Formamide on a 3730 DNA Analyzer, and fragment size was determined using GeneMapper Software Version 4.0 software (Applied Biosystems). To confirm allele length, ∼ 8% of samples were validated by sequencing with BigDye Terminator Version 1.1 on a 3730 DNA Analyzer (Applied Biosystems).
α-globin gene deletion or duplication status was determined by multiplex PCR assays or Mulitplex Ligation-Dependent Probe Amplification (MLPA). Two-tube multiplex PCR was performed adapting previously published primers to determine α-globin deletion or duplication status.22,23 PCR was carried out in 25-μL reactions containing 200μM of dNTP, 1.5mM MgCl2, 2.5 U HotStarTaq DNA polymerase with 1 times Q-solution (QIAGEN), 200 ng of genomic DNA, and set 1 or set 2 primers (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Amplification was performed with an initial heat activation step of 15 minutes at 96°C followed by 30 cycles of 98°C for 45 seconds, 65°C (set 1) or 60°C (set 2) for 90 seconds, and 72°C for 150 seconds, and a 5-minute final extension step at 72°C. PCR bands were separated and sized on 1% agarose gels.
The MLPA was carried out using SALSA MLPA kit P140 HBA (MRC-Holland) following the manufacturer's instructions. Products of the MLPA reaction were analyzed on the 3730 Genetic Analyzer (Applied Biosystems) using GeneScan 500 LIZ Size Standard (Applied Biosystems). Data were analyzed using GeneMapper Version 4.0 (Applied Biosystems) and Coffalyser (MRC-Holland). MLPA and multiplex PCR assay results were cross-validated for 10% of the samples (59 persons with no α-globin deletion, 18 persons with 1 gene deletion, and 16 persons with 2 gene deletions).
(GT)n repeat classification
For initial analyses, the observed HMOX1 (GT)n repeat sizes were divided into 2 allele classes: short (S) with ≤ 25 repeats and long (L) with > 25 repeats. A sensitivity analysis testing a range up to 30 repeats confirmed that a cutoff of ≤ 25 repeats was the best fit for short alleles. To explore the importance of the observed trimodal distribution among this population, posthoc analyses grouped alleles into 3 classes: short (S) with 25 or fewer repeats, medium (M) with 26 to 34 repeats, and long (L) with 35 or more repeats.
Haplotyping
Statistical analysis
All other analyses were conducted using SAS Version 9.2 (SAS Institute). χ2 and Student t tests were used to assess the statistical significance of variations in the distribution of demographic and clinical characteristics (age, sex, asthma diagnosis, steady-state white blood count, steady-state hemoglobin, steady-state reticulocytes, total bilirubin, fetal hemoglobin, and α-globin gene deletion status) by repeat length classification, and all P values for these tests were 2-tailed. For children with more than 1 bilirubin measurement, the highest value during the 3-year period was used for the analysis. The log transformation of white blood count and fetal hemoglobin was used to approximate normality.
The incidence of hospitalization for ACS and pain episodes according to allele length classification was calculated using negative binomial regression models, with a scale parameter estimated by maximum likelihood. These findings were confirmed using both Poisson regression with correction for overdispersion and zero-inflated Poisson regression. The initial models included all aforementioned demographic and clinical characteristics as covariates; only those with P values < .05 were retained in the final model. Thus, adjusted rate ratios were calculated using models that controlled for sex, asthma diagnosis, percentage of fetal hemoglobin, and α-globin gene deletion status.
Results
Demographics
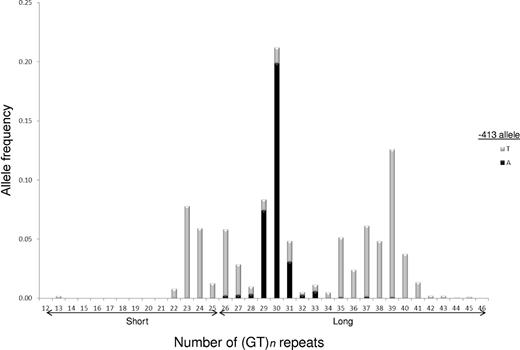
A total of 942 children with SCD had complete clinical data and DNA available for analysis. The HMOX1 (GT)n repeat was highly polymorphic, and the distribution of allele sizes appeared trimodal among those in this cohort. We observed 28 different alleles with the number of dinucleotide repeats varying from 13 to 45 (Figure 1). In addition to peaks at 23 and 30 repeats, there was a third large peak centered near 39 repeats among our patient population. We analyzed our results with only 2 allele categories (S and L) and a conservative cutoff for S alleles of 25 or fewer dinucleotide repeats.
Trimodal HMOX1 (GT)n repeat distribution among 942 participants with SCD in the SIT Trial. Allele lengths were scored as number of dinucleotide repeats and ranged from 13 to 45 repeats. Individual (GT)n alleles assigned to −413 T (gray bars) or A (black bars) chromosomes by PHASE 2.1 haplotype estimation. Initial analyses were performed by grouping observed HMOX1 (GT)n repeat sizes into 2 allele classes: short (S) with ≤ 25 repeats and long (L) with > 25 repeats.
Trimodal HMOX1 (GT)n repeat distribution among 942 participants with SCD in the SIT Trial. Allele lengths were scored as number of dinucleotide repeats and ranged from 13 to 45 repeats. Individual (GT)n alleles assigned to −413 T (gray bars) or A (black bars) chromosomes by PHASE 2.1 haplotype estimation. Initial analyses were performed by grouping observed HMOX1 (GT)n repeat sizes into 2 allele classes: short (S) with ≤ 25 repeats and long (L) with > 25 repeats.
Characteristics of children with SCD and laboratory values were compared between S/S repeat homozygous persons and all others (Table 1). No statistically significant (P < .05) associations were noted in the distribution of age, sex, asthma diagnosis, steady-state white blood count, steady-state hemoglobin, steady-state reticulocytes, and total bilirubin among children with SCD and the S/S (GT)n genotype compared with all other genotypes. Higher percent of fetal hemoglobin was significantly associated with S/S genotype and was retained in the final models.
Demographic and clinical characteristics of children in the SIT Trial by (GT)n genotype class
| Characteristic* . | S/S† (n = 35) . | S/L + L/L† (n = 907) . | P . | ||
|---|---|---|---|---|---|
| N . | Mean or % . | N . | Mean or % . | ||
| Age at registration | 35 | 9.2 | 907 | 9 | .79 |
| Male | 35 | 60 | 914 | 51.5 | .32 |
| Ever had asthma | 35 | 34.3 | 907 | 25.1 | .22 |
| Steady-state white blood count, count/μL | 34 | 11 811 | 894 | 12 648 | .39‡ |
| Steady-state hemoglobin, g/dL | 35 | 8 | 906 | 8.1 | .81 |
| Steady-state reticulocytes | 32 | 13.7 | 889 | 12 | .08 |
| Total bilirubin, mg/dL | 33 | 3.7 | 835 | 3.8 | .94 |
| Fetal hemoglobin | 32 | 14 | 895 | 12.7 | .01‡ |
| Characteristic* . | S/S† (n = 35) . | S/L + L/L† (n = 907) . | P . | ||
|---|---|---|---|---|---|
| N . | Mean or % . | N . | Mean or % . | ||
| Age at registration | 35 | 9.2 | 907 | 9 | .79 |
| Male | 35 | 60 | 914 | 51.5 | .32 |
| Ever had asthma | 35 | 34.3 | 907 | 25.1 | .22 |
| Steady-state white blood count, count/μL | 34 | 11 811 | 894 | 12 648 | .39‡ |
| Steady-state hemoglobin, g/dL | 35 | 8 | 906 | 8.1 | .81 |
| Steady-state reticulocytes | 32 | 13.7 | 889 | 12 | .08 |
| Total bilirubin, mg/dL | 33 | 3.7 | 835 | 3.8 | .94 |
| Fetal hemoglobin | 32 | 14 | 895 | 12.7 | .01‡ |
Steady-state levels defined at routine clinical well-visit.
HMOX1 (GT)n allele classes defined by number of repeats: S ≤ 25 repeats; and L > 25 repeats.
P value based on Student t test of log differences.
−413A > T promoter SNP
HMOX1 −413A > T SNP genotyping results were in Hardy-Weinberg equilibrium and consistent with results for the Yoruban population in Ibadan, Nigeria, reported by the International HapMap Project.26 The frequency of the minor allele (A) among this population was 32% (Table 2). No association between the presence of the HMOX1 −413A > T polymorphism and rate of hospitalization for pain or ACS was observed (data not shown).
Distribution of HMOX1 polymorphisms and α-globin gene deletion status among 942 children in the SIT Trial by genotype and allele class
| −413A > T (rs2071746) . | (GT)n(rs3074372): S/L . | α-globin status . | |||
|---|---|---|---|---|---|
| Genotype* . | % . | Genotype† . | % . | Genotype‡ . | % . |
| AA | 11.6 | S/S | 3.7 | αα/αα | 58.2 |
| AT | 42.1 | S/L | 25.2 | −α/αα | 36.6 |
| TT | 46.3 | L/L | 71.1 | −α/-α | 5.2 |
| −413A > T (rs2071746) . | (GT)n(rs3074372): S/L . | α-globin status . | |||
|---|---|---|---|---|---|
| Genotype* . | % . | Genotype† . | % . | Genotype‡ . | % . |
| AA | 11.6 | S/S | 3.7 | αα/αα | 58.2 |
| AT | 42.1 | S/L | 25.2 | −α/αα | 36.6 |
| TT | 46.3 | L/L | 71.1 | −α/-α | 5.2 |
−413A > T results are in Hardy-Weinberg equilibrium (P = .369).
(GT)n allele classes defined by number of repeats: S ≤ 25 repeats; and L > 25 repeats.
Persons with αα/ααα genotypes carried no gene deletions and were grouped with αα/αα.
HMOX1 promoter polymorphism joint distribution
We examined the cosegregation of the HMOX1 −413A > T SNP and (GT)n repeat variants. All persons who were homozygous for 2 S repeat alleles also were homozygous for the T allele at the −413 SNP (Table 3). Haplotype estimation revealed that the −413A allele was never found on the same chromosome as an S repeat allele among those in this cohort (Figure 1).
Cosegregation of HMOX1 promoter polymorphisms among 942 children in the SIT Trial
| — . | S/S, % . | S/L, % . | L/L, % . |
|---|---|---|---|
| Genotype* | |||
| AA | 0.0 | 0.0 | 16.3 |
| AT | 0.0 | 40.0 | 45.1 |
| TT | 100.0 | 60.0 | 38.6 |
| Haplotype† | |||
| T-L | 51.0 | — | — |
| A-L | 32.7 | — | — |
| T-S | 16.3 | — | — |
| — . | S/S, % . | S/L, % . | L/L, % . |
|---|---|---|---|
| Genotype* | |||
| AA | 0.0 | 0.0 | 16.3 |
| AT | 0.0 | 40.0 | 45.1 |
| TT | 100.0 | 60.0 | 38.6 |
| Haplotype† | |||
| T-L | 51.0 | — | — |
| A-L | 32.7 | — | — |
| T-S | 16.3 | — | — |
— indicates not applicable.
Distribution of AA, AT, and TT genotypes in S/S, S/L, and L/L persons.
Summary of haplotype estimation results: S ≤ 25 (GT)n repeats; and L > 25 (GT)n repeats.
α-globin gene locus
Overall, 42% of the participants carried at least 1 α-globin gene deletion or duplication (Table 2). As expected, the −α3.7 single gene deletion was the most prevalent mutation in this cohort, accounting for > 98% of the structural variants detected at the α-globin gene locus. In addition, 2 persons carried the −α4.2 single gene deletion and 6 persons carried an α-globin gene triplication (αα/ααα).
Factors associated with vaso-occlusion
Sex, asthma diagnosis, percentage of fetal hemoglobin, and α-globin gene deletion status were found to influence significantly the incidence of ACS or pain episodes among children in the SIT Trial and were retained as covariates in the final models (Table 4). Percent fetal hemoglobin was treated as a continuous variable, with higher levels associated with a lower rate of ACS. Consistent with previous reports, males and participants with a positive asthma diagnosis had higher rates of vaso-occlusive crises. Persons carrying 1 or 2 α-globin gene deletions had higher rates of pain episodes (P = .03 and P = .02, respectively) and no statistical difference in the incidence rate of ACS.
Factors associated with vaso-occlusive crises in the SIT Trial
| Covariate . | ACS . | Pain . | ||
|---|---|---|---|---|
| Rate . | P . | Rate . | P . | |
| % fetal hemoglobin* | < .0001 | .23 | ||
| Sex | ||||
| Male | 0.17 | .005 | 0.68 | .07 |
| Female | 0.12 | 0.58 | ||
| Asthma diagnosis | ||||
| Yes | 0.21 | < .0001 | 0.74 | .02 |
| No | 0.12 | 0.59 | ||
| α-globin genotype† | ||||
| αα/αα | 0.15 | 0.56 | ||
| α/αα | 0.14 | .41 | 0.69 | .03 |
| α/-α | 0.08 | .07 | 0.88 | .02 |
| Covariate . | ACS . | Pain . | ||
|---|---|---|---|---|
| Rate . | P . | Rate . | P . | |
| % fetal hemoglobin* | < .0001 | .23 | ||
| Sex | ||||
| Male | 0.17 | .005 | 0.68 | .07 |
| Female | 0.12 | 0.58 | ||
| Asthma diagnosis | ||||
| Yes | 0.21 | < .0001 | 0.74 | .02 |
| No | 0.12 | 0.59 | ||
| α-globin genotype† | ||||
| αα/αα | 0.15 | 0.56 | ||
| α/αα | 0.14 | .41 | 0.69 | .03 |
| α/-α | 0.08 | .07 | 0.88 | .02 |
P values derived from the regression of ACS or pain on the log transformation of fetal hemoglobin.
Persons with αα/ααα genotypes carried no gene deletions and were grouped with αα/αα for analysis.
Repeat length and ACS
The association between short allele length and a decreased rate of hospitalization for ACS fit a recessive genetic model. Children with SCD and the S/S (GT)n genotype had 0.04 ACS events per patient year, compared with 0.15 events per patient year for children with SCD and S/L or L/L (GT)n genotypes; the unadjusted rate ratio for this comparison was 0.26 (0.09-0.75; Table 5). After adjusting for sex, asthma diagnosis, percentage of fetal hemoglobin, and α-globin deletion status, the adjusted rate ratio was 0.29 (95% confidence interval, 0.10-0.84).
Estimates of the per patient-year incidence of ACS and pain that required hospitalization among children in the SIT Trial according to (GT)n genotype class
| . | S/S* . | S/L + L/L* . | Unadjusted rate ratio . | 95% CI . | Adjusted rate ratio† . | 95% CI . |
|---|---|---|---|---|---|---|
| ACS | 0.038 | 0.147 | 0.26 | 0.09-0.75 | 0.28 | 0.10-0.81 |
| Pain | 0.600 | 0.628 | 0.96 | 0.60-1.51 | 1.03 | 0.65-1.65 |
| . | S/S* . | S/L + L/L* . | Unadjusted rate ratio . | 95% CI . | Adjusted rate ratio† . | 95% CI . |
|---|---|---|---|---|---|---|
| ACS | 0.038 | 0.147 | 0.26 | 0.09-0.75 | 0.28 | 0.10-0.81 |
| Pain | 0.600 | 0.628 | 0.96 | 0.60-1.51 | 1.03 | 0.65-1.65 |
HMOX1 (GT)n allele classes defined by number of repeats: S ≤ 25 repeats; and L > 25 repeats.
Adjusted for sex, ever had asthma, percentage of fetal hemoglobin, and α-globin gene deletion status.
To refine the possible clinical importance of various allele sizes, this association was retested after further dividing the (GT)n alleles into 3 classes (S, M, and L) based on the observed trimodal distribution (Figure 1). The protective effect remained restricted to the S/S (GT)n genotype class, and we found no evidence for a difference in the rate of hospitalization for ACS with M versus L class repeat alleles (Table 6).
Adjusted rate ratios for ACS for each genotype class versus all others
| Genotype* . | % . | Adjusted rate ratio† . | 95% CI . |
|---|---|---|---|
| S/S | 3.7 | 0.28 | 0.10-0.81 |
| S/M | 14.0 | 0.97 | 0.67-1.39 |
| S/L | 11.2 | 1.00 | 0.68-1.48 |
| M/M | 22.8 | 0.92 | 0.68-1.24 |
| M/L | 33.0 | 1.15 | 0.89-1.48 |
| L/L | 15.3 | 1.09 | 0.78-1.53 |
| Genotype* . | % . | Adjusted rate ratio† . | 95% CI . |
|---|---|---|---|
| S/S | 3.7 | 0.28 | 0.10-0.81 |
| S/M | 14.0 | 0.97 | 0.67-1.39 |
| S/L | 11.2 | 1.00 | 0.68-1.48 |
| M/M | 22.8 | 0.92 | 0.68-1.24 |
| M/L | 33.0 | 1.15 | 0.89-1.48 |
| L/L | 15.3 | 1.09 | 0.78-1.53 |
(GT)n allele classes defined by number of repeats: S ≤ 25 repeats, M = 26-34 repeats, and L ≥ 35 repeats.
Adjusted for sex, ever had asthma, percentage of fetal hemoglobin, and α-globin gene deletion status.
Repeat length and pain
The incidence of pain requiring hospitalization was not significantly different among children with SCD and the S/S (GT)n genotype and those with other genotypes (0.60 and 0.63 pain events per patient year, respectively; Table 5). After adjustment for sex, asthma diagnosis, percentage fetal hemoglobin, and α-globin gene deletion status, the association between genotype class and rate of hospitalization for pain remained nonsignificant. Further analyses after dividing the alleles into 3 classes as described in “Repeat length and ACS” revealed no significant association between pain and any of the genotype classes (data not shown).
Discussion
Despite the clinical importance of vaso-occlusive episodes in pain and ACS, there remains limited insight into their underlying etiology and pathogenesis. The evaluation of genetic variation and its association with incidence of vaso-occlusion pain or ACS episodes is one strategy for elucidating their etiology and pathogenesis. In this study, we demonstrated that genetic variation in the inducible form of heme oxygenase, HO-1, was associated with the incidence of ACS episodes. Specifically, children homozygous for short (GT)n repeat alleles had a significantly lower rate of hospitalization per patient year for ACS after adjusting for sex, asthma diagnosis, fetal hemoglobin, and α-globin gene deletion status.
The HMOX1 (GT)n promoter microsatellite has been evaluated for its role in a number of clinical outcomes, including cardiovascular and pulmonary diseases.8,27,28 This study is the first to test (GT)n repeat alleles for clinical associations among a large population of persons affected with ongoing hemolysis, such as SCD. An efficient heme oxygenase response might be particularly important to protect persons with SCD from the damaging effects of excess heme released by high rates of chronic red blood cell sickling and lysis.
A growing consensus exists that persons with shorter (GT)n repeat alleles might benefit by having a stronger inducible heme oxygenase response; however, the specific classification of allele sizes and groups has varied among studies.8,27 A cutoff of ≤ 25 repeats commonly has been used to define short alleles, but this definition has ranged from 23 to 30 dinucleotide repeats.27 In addition, instead of comparing only short versus long alleles, many studies have classified (GT)n repeats into 3 categories: short (S), medium (M), and long (L). We performed our initial analyses by grouping alleles into short and long categories before further dividing the alleles by adding a medium group, to examine more closely the effect of allele size in posthoc analyses; however, our data suggested no clinically significant difference between M and L alleles among this population, and no protective effect was observed, even among persons with S/M genotypes who carry only a single medium allele.
The HMOX1 −413A > T SNP has been studied less than the (GT)n repeat; however, this polymorphism also has been associated with variable promoter activity and disease outcome in some studies.9,29 We found no association between this SNP and any clinical outcome tested, including rates of hospitalization for pain or ACS. Among this cohort, no patient with an AA genotype at −413 of the HMOX1 gene also carried a short repeat allele. Indeed, haplotype analysis indicated that the peak at 30 (GT)n repeats was segregating mainly on −413A allele chromosomes. Our data suggested that, among this population of persons with SCD, the clinical significance of genetic variation in the HMOX1 promoter might have been restricted to the (GT)n repeat.
The pathophysiology of ACS is not completely understood, and multiple etiologies, both infectious and noninfectious, have been implicated in the onset and severity of the episode.17 Although sickle cell-related pain episodes are an important risk factor for ACS and have been reported to occur shortly before diagnosis among both children and adults,16 we did not observe an association between genetic variation in the HMOX1 promoter and rate of hospitalization for pain.
The inducible heme oxygenase-1 pathway plays a central cytoprotective role in defending against chronic and acute inflammatory conditions.27,28 Accumulation of free heme is damaging, as excess heme is a potent inducer of vascular inflammation and is a known generator of reactive oxidative species. The catabolism of heme by heme oxygenases removes a major source of damaging reactive oxidative species and produces anti-inflammatory and cytoprotective molecules, including biliverdin and carbon monoxide.5 Studies in mouse models of SCD have demonstrated that increases in levels of heme oxygenase-1, and products of heme degradation can ameliorate and prevent vascular inflammation and vaso-occlusion7 ; moreover, recent studies have suggested that protein-free heme triggers acute lung injury in transgenic SCD mice.30
Among persons with low levels of free heme in their vasculature, induction of HO-1 expression and activity serves to return the vasculature to a balanced steady state after an insult.31 Persons with SCD have high rates of hemolysis that might be elevated further during vaso-occlusive pain episodes and ACS.32 In SCD, the state of chronic hemolysis places overwhelming demand on the anti-inflammatory, antioxidant properties of the HO-1 response. We postulated that the efficacy of the HO-1 response among these persons is determined primarily by genetic variation, namely, (GT)n promoter repeat length, and this genetic variation of the HMOX1 gene is associated with a key SCD manifestation. In support of this hypothesis, our data showed that persons with short HMOX1 (GT)n promoter repeat alleles had decreased rates of hospitalization for ACS.
Another unique aspect of this study was that it described HMOX1 (GT)n genotyping among the largest population to date of persons of African ancestry; previous studies were performed primarily among either white or Asian populations. We observed a unique distribution of (GT)n repeat sizes in this study compared with nonblack populations. Specifically, we identified a pattern of greater genetic variation that was shifted toward longer repeats.
That enrichment in longer alleles would occur among African populations, despite a disadvantage for persons with HbSS, might simply reflect increased diversity from microsatellite evolution,33,34 but it raises the possibility that HMOX1 repeat alleles are under strong selective pressure in regions where malaria is endemic.35 Further investigations of HMOX1 genotypes in other hemolytic disorders, combined with gene expression studies in malaria-endemic regions, will be necessary to clarify the role of genetic variation among these populations.
A limitation of this study was that no measurements of plasma HO-1 activity could be made in participants to assess directly the association between enzyme activity and risk of clinical outcomes, including rates of hospitalization for pain and ACS. However, the preponderance of the existing literature is that persons with lower numbers of HMOX1 (GT)n promoter repeats have higher inducible heme oxygenase expression.11,12,36 Additional functional studies of mRNA and enzyme activity will be necessary to better characterize the heme oxygenase response in persons with SCD. Given the relatively low rate of hospitalization for ACS and frequency of the protective HMOX1 genotype (S/S), ∼ 4% of the entire cohort, a study to validate our findings with functional measurements would probably require an equally large cohort of ∼ 1000 persons with SCD followed for at least 3 years. Mechanistic studies interrogating the role of HMOX1 genotype and heme metabolism in experimentally induced ACS in transgenic SCD mice or other experimental approaches will be required to better elucidate whether there is a causal relationship between heme oxygenase activity and ACS.
In conclusion, children with SCD who carried short HMOX1 (GT)n repeat promoter alleles had a significantly lower rate of hospitalization for ACS. The protective effect was restricted to the homozygous S/S repeat genotype class, and our data suggested a repeat size threshold effect, with no functional difference between M and L alleles among these persons. To our knowledge, this is the first study to examine the role of genetic variation in HMOX1 in SCD, a chronic hemolytic disorder. Our results implicate a role for the heme oxygenase-heme axis in the etiology of ACS and highlight the need for future studies to interrogate genetic variation in this pathway as one of potential mechanisms for acute lung injury. ACS is a leading cause of death among persons with SCD. Greater understanding of the role of the HMOX1 response in the setting of an exacerbation of chronic hemolysis may elucidate targeted strategies to prevent or attenuate life-threatening ACS episodes.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the families and children with SCD who were participants, the SIT Trial study coordinators, and laboratory members in the Division of Blood Disorders and Division of Pediatric Hematology.
This work was supported by National Heart, Lung, and Blood Institute (RO1-HL079937, M.R.D.; and 1U54HL090515, J.F.C.), Burroughs Wellcome Foundation (M.R.D.), National Institute of Neurological Disorders and Stroke (U01-NS-042804; E.A.B.-C.), and Center for Translational Science Award (M01-RR00052, J.F.C.; and K02HL088026, S.F.O.-A.).
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
National Institutes of Health
Wellcome Trust
Authorship
Contribution: C.J.B. designed the experiments, analyzed the data, and wrote the manuscript; S.L.B. analyzed the data and wrote the manuscript; D.E., M.E.P., A.B.P., J.D., H.A.T., G.Y., and K.J. performed the laboratory experiments at the HMOX1 and α-globin gene loci; E.A.B.-C., J.F.C., and M.R.D. (as part of the SIT Biologic Repository) collected key reagents and data, designed the experiments, and wrote the manuscript; and S.F.O.-A and W.C.H. designed the experiments and wrote the manuscript.
Conflict-of-interest disclosure: J.F.C. has received an honorarium and travel expenses in the past and presently receives salary support through Johns Hopkins for providing consultative advice to Adventrx Pharmaceuticals regarding a proposed clinical trial of an agent for treating vaso-occlusive crisis in SCD. The remaining authors declare no competing financial interests.
Correspondence: Michael R. DeBaun, Vanderbilt, Meharry, Matthew Walker Center of Excellence in Sickle Cell Disease, Vanderbilt University School of Medicine, 215 Light Hall, Nashville, TN 37232; e-mail: m.debaun@vanderbilt.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal