Abstract
Surprisingly, the role(s) of eosinophils in health and disease is often summarized by clinicians and basic research scientists as a pervasive consensus opinion first learned in medical/graduate school. Eosinophils are rare white blood cells whose activities are primarily destructive and are only relevant in parasitic infections and asthma. However, is this consensus correct? This review argues that the wealth of available studies investigating the role(s) of eosinophils in both health and disease demonstrates that the activities of these granulocytes are far more expansive and complex than previously appreciated. In turn, this greater understanding has led to the realization that eosinophils have significant contributory roles in a wide range of diseases. Furthermore, published studies even implicate eosinophil-mediated activities in otherwise healthy persons. We suggest that the collective reports in the literature showing a role for eosinophils in an ever-increasing number of novel settings highlight the true complexity and importance of this granulocyte. Indeed, discussions of eosinophils are no longer simple and more often than not now begin with the question/statement “Did you know …?”
Introduction
Hematology and medical text-books (eg, Hematology: Basic Principles and Practice, 5th edition) often portray eosinophils with 3 basic features: (1) they are nonspecific destructive and cytotoxic cells; (2) eosinophils are an omnipresent cellular infiltrate of the asthmatic lung; and (3) eosinophils are a necessary and ubiquitous host defense against helminthic parasite infections.1,2 Thus, although this physician-scientist view of eosinophils is beginning to change (eg, Wintrobe's Clinical Hematology, 12th edition), the widely held clinical perception of these granulocytes is that they are “bad,” except for parasite clearance in patients, and even then, their function is only that of an end-stage destructive cell. That is, they are part of an innate host defense strategy that recruits these cells to sites of parasitic infection or as part of the dysregulated immune responses in the asthmatic lung, where they mediate nonspecific destruction of all things (pathogen and host tissue alike). Consequently, the tissue/organ pathology induced by their recruitment is nothing more than collateral damage of a mission accomplished (ie, host defense). We will show that this perspective is outdated and problematic with a virtual explosion of recent studies highlighting previously unknown and/or underappreciated eosinophil effector functions that emphasize a changing view of eosinophils in health and disease.
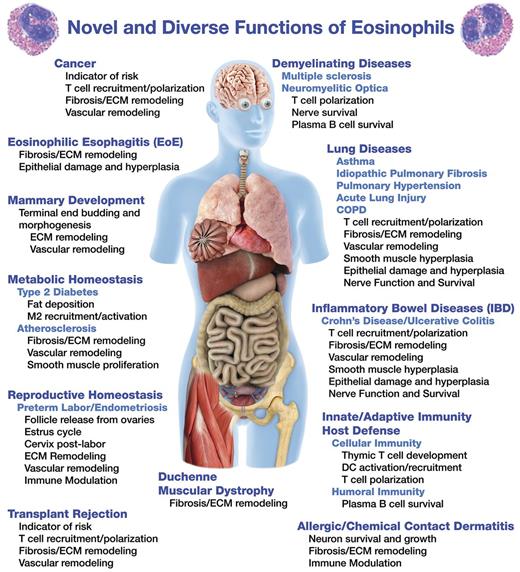
This confounding paradigm-shift begs the question: What are these cells really doing? This review highlights studies that suggest diverse and novel eosinophil effector functions (Figure 1). Many of these functions are contained within our recently suggested paradigm that we have described as the “LIAR hypothesis,” suggesting that instead of destructive end-staged effector cells, eosinophils are actually important regulators of local immunity and remodeling/repair.3 This hypothesis predicts the widening scope of eosinophil effector functions and suggests contributory roles of these granulocytes in both health and disease. Our goal in this review is simply to continue this “conversation” and summarize many of these new eosinophil effector functions and to note the novel roles these cells may have in the maintenance of homeostasis.
Eosinophils: agents of local tissue/organ immune regulation
Eosinophils contain a full complement of mediators necessary to regulate specifically both innate and adaptive immune responses. Many excellent review articles4,5 describe in great detail immune mediators released by eosinophils and will not be reviewed in-depth here. In brief, eosinophils express Th2 cytokines (eg, IL-4, IL-5, IL-9, IL-13, and IL-25), Th1 cytokines (IL-12 and IFN-γ), acute proinflammatory cytokines (TNF-α, IL-1β, IL-6, and IL-8), immune inhibitory cytokines (eg, TGF-β and IL-10), and express receptors for many of these cytokines as well.6,7 In addition, eosinophils express molecules that directly modify T-cell activities through Notch pathways8 as well as costimulatory molecules (eg, CD80/86) and MHC class II that allow eosinophils to function as antigen-presenting cells.9 Innate immune activities of eosinophils include expression of pattern recognition receptors, such as Toll-like receptors 1-5, 7, and 9, nucleotide oligomerization domains 1 and 2, Dectin-1, and receptor for advanced glycation end products, which recognize pathogen-associated molecular patterns or danger-associated molecular patterns.10,11 Eosinophil granule proteins are also capable of binding pattern recognition receptors in an autocrine and paracrine manner to induce cellular activation. Moreover, eosinophils express a host of immune modulating chemokines and adhesion molecules,12 complement receptors,13 and lipids and their receptors.14 In several examples cited below, these previously underappreciated immune regulatory capabilities of eosinophils are highlighted as of potentially significant importance in both health and disease.
Eosinophils: tissue remodeling and repair
Remodeling and repair are generally associated with permanent structural and functional change from the original cellular and physiologic capacity of a cell, tissue, or organ. Thus, remodeling and repair includes, and is not limited to, extracellular matrix breakdown and reformation, cellular transdifferentiation (eg, transition of fibroblasts to myofibroblasts or Clara cells to goblet cells in the respiratory epithelium), apoptosis/necrosis, cell proliferation, and altered cellular activation. These events are initiated by the release of growth factors, cytokines, chemokines, enzymes, lipid mediators, and reactive oxygen species from the tissue or infiltrating inflammatory cells. Eosinophils have been demonstrated to express and release both mediators of epithelial-mesenchymal transition, such as TGF-β, basic fibroblast growth factors, platelet-derived growth factor, matrix metalloproteases, vascular endothelial growth factors4,5,11 as well as other repair/remodeling factors, such as nerve growth factors, neuropeptides,15 and cytokines such as IL-1β and IL-6.6,7 Furthermore, eosinophil release of secondary granule proteins, eicosanoids, leukotrienes, reactive oxygen species, and cell-cell signaling molecules have been hypothesized to contribute to remodeling events in both health (eg, bone metabolism16 ) and various disease states. Manifestations of these activities in disease states include increased fibrosis, vascular leakage, angiogenesis, epithelial desquamation, epithelial metaplasia, and smooth muscle hypertrophy.17-19 As such, reports in the literature have linked eosinophils with the remodeling events and structural changes occurring in prominent eosinophil-associated conditions as well as more obscure (ie, not necessarily eosinophil-associated) diseases.
Classic eosinophil diseases with novel roles
Eosinophils and parasites: not necessarily host defense but instead symbiotic partners
Eosinophils are a common proinflammatory cell infiltrate during helminth infection and are often considered necessary for parasite killing.1 For example, animal model studies have noted that eosinophils are directly recruited within hours to days after tissue infiltration of parasites and appear to participate in the killing of the parasite through the release of toxic eosinophil effector mediators, such as major basic protein20 and reactive oxygen species.21 However, other studies using eosinophil-deficient mice (eg, ΔdblGATA-122 and PHIL23 ) have demonstrated virtually no difference in worm burden in the presence or absence of eosinophils.24 These data suggested that, although eosinophils have a more active “killing” role in some parasitic infections,25 other nonlethal activities of significance nonetheless exist, leading investigators to underappreciate the true extent of eosinophil effector functions.
Recent studies by Gebreselassie et al26 and Fabre et al27 in the mouse may have provided a solution to this quandary, suggesting a unique role for eosinophils not in parasite killing but instead for parasite survival. Specifically, Trichinella spiralis has a 2-stage life cycle whereby newborn larvae convert a muscle cell into a nurse cell for the developing parasite. This nurse cell is surrounded by macrophages in a manner mimicking granuloma formation required by all parasites,28 allowing the parasite to remain quiescent in its host for months to years. In the absence of eosinophils, the number of nurse cells is dramatically reduced in infected mice.27 Furthermore, in the absence of eosinophils, infiltrating Th2 T cells are reduced and recruited macrophages have enhanced iNOS expression,26 suggesting that, in the absence of eosinophil-mediated Th2 cytokine production, iNOS-expressing inflammatory macrophages (M1 type) outnumber the alternatively activated macrophages (M2 type). The resulting excessive host inflammatory responses led to the death of the resident parasite and, in turn, significant muscle pathology/necrosis. The failure of nurse cells to thrive in eosinophil-deficient mice was rescued by intravenous transfer of eosinophils into the mice,26 demonstrating a direct role for eosinophils in aiding helminth survival while at the same time dampening the inflammatory response of the host. The mechanisms of this eosinophil-mediated macrophage regulation remain to be defined, yet studies by Stolarski et al that demonstrated eosinophil release of IL-4 and IL-13 polarized macrophage toward an M2 phenotype suggest a mechanism by which eosinophils directly modulate local Th2 polarization conditions to protect the resident parasite.29 Consequently, eosinophils may be interpreted as being part of a strategy to achieve homeostasis allowing the parasite to reside in the host with limited pathologic consequences to either the parasite or host, a conclusion that represents a return to the conclusions of earlier studies suggesting that recruited eosinophils contain (ie, limit) ongoing inflammatory events.30
Eosinophils and asthma: not necessarily destructive effector cells mediating lung pathology but instead modulators of pulmonary immune responses to allergen
Eosinophils have been viewed as omnipresent inflammatory cells whose role in asthma, although not clearly defined, has been characterized as destructive.2 As a result, their presence in airways was suggested to increase and/or cause the symptoms occurring in asthma patients (eg, airways hyper-responsiveness). This suggestion was also supported in many mouse models of allergic respiratory inflammation, including a transgenic model of severe asthma where induced pulmonary pathology was directly linked with one or more eosinophil-mediated effector functions.31 However, improvement of symptoms in asthma patients after therapies targeting eosinophils have not been linear and have been met with limited success,32,33 suggesting that a much more complex relationship between eosinophils and induced lung pathology exists. A potentially greater understanding of how eosinophils may contribute to asthma (ie, allergic respiratory inflammation) was achieved with the demonstration that eosinophils have the ability to regulate immune responses through direct effects on T-cell activities in mouse models of asthma. A brief review of these studies provides the necessary context for the evolution of this new paradigm correlating the spatial distribution of eosinophils and their immune regulatory roles.
Several reports have proposed and tested various mechanisms that would explain the potential modulation of T-cell activities by eosinophils. The unique ability(ies) of eosinophils to elicit the local recruitment/accumulation of allergen-specific effector T cells on allergen exposure were demonstrated in 2 independent studies.34,35 In addition, eosinophils have been uniquely shown to contribute to the activation of antigen-specific memory T cells (promoting their proliferation) and function as agents that polarize on-going T-cell responses. This ability of eosinophils to promote T-cell activation/proliferation (ie, display dendritic cell [DC]-like activities) was initially outlined in studies using both mouse models of asthma as well as in vitro assays on patient samples. Specifically, these studies suggested that eosinophils may function as costimulatory cells acting on both primed and naive T cells.9 Clearly, mechanistic studies involving human asthma subjects have lagged behind the pace at which these insights have been revealed in the mouse. For example, mouse eosinophils have been shown to process and present antigen via MHC class II36,37 and induce Th2 cytokine production from antigen-specific CD4+ T cells.36,38 Moreover, eosinophils were demonstrated to migrate to lung draining lymph nodes in mice with similar kinetics to DCs and reside in the T-cell rich zone.39,40 It is noteworthy that more recent studies in mouse models of asthma have since demonstrated that, although eosinophils have the capacity to act as antigen-presenting cells, DCs are still likely to be the predominant MHC class II antigen-presenting cell that results in proliferation of T cells41,42 with eosinophils contributing to the polarization of the in vivo immune responses occurring in the lung.43
Jacobsen et al have recently identified an additional eosinophil-mediated effect on T-cell activities, suggesting that eosinophils are not replacements for DCs but instead are third party cells necessary for DC-mediated T-cell proliferation and subsequent Th2 polarization.43 These investigators expanded on earlier studies by Niu et al44 and Hammad et al42 demonstrating the requirement of a non-DC (but MHC class II expressing) cell that was necessary for the T-cell proliferation and polarization induced by DC-mediated antigen presentation. In particular, Jacobsen et al showed that adoptive transfer of eosinophils into eosinophil-deficient animals during allergen challenge resulted in recruitment of antigen-presenting myeloid DCs to those lymph nodes and also in the subsequent Th2 polarized character of the immune response. Significantly, these data demonstrated that eosinophils polarize the T-cell response to Th2 by a unique pathway; it appeared that eosinophils promoted the suppression of Th1 and, in particular, Th17 responses. Although the mechanisms have not been defined, eosinophil-released cytokines may be directly affecting T cell or DC responses through release of Th2 cytokines (eg, IL-4 and IL-25) or suppressive mediators (eg, IL-10, indoleamine-2 3-dioxygenase [IDO], and TGF-β). In addition, other studies have demonstrated that human eosinophils are capable of modulating DC migration and activation.45 Thus, in mice, and possibly humans, eosinophils may contribute to the polarization of immune responses by directly modulating T cells and/or indirectly by influencing DC-mediated activities.
It is interesting to speculate that the blockade of eosinophil-mediated immune regulation may also provide an explanation for the efficacy of inhaled corticosteroids and anti–IL-5–based treatments in both human subjects with asthma (ie, mepolizumab32,33 and benralizumab46 ) and mouse models of allergic respiratory inflammation.47,48 That is, in both species the eosinophil-targeting effects of these agents may not only prevent tissue damaging effector functions but also disrupt eosinophil-mediated immune regulatory pathways. Clearly, further studies are necessary in both asthma patients and mouse models to confirm the potential commonality of eosinophil activities in allergic pulmonary diseases.
Eosinophilic esophagitis
Eosinophilic esophagitis is a significant cause of dysphagia and food impaction in adults and is also linked with vague symptoms previously associated with gastroesophageal reflux disease in children.49 Predictably, eosinophilic esophagitis has been mechanistically linked in both human subjects50 and mouse models51 with eosinophil agonists, such as IL-5 and eotaxin chemokines. Although the consensus in the literature is that eosinophils are omnipresent in this disease and likely to have one or more causative role(s), the importance of their presence or individual activities has remained unresolved.
Novel eosinophil roles in health
Eeosinophils and plasma cell survival
Long-term memory in humoral immunity is acquired by the survival and continued function of plasma B cells to generate antibody against pathogens. A novel study by Chu et al demonstrated a role for eosinophils in plasma B-cell survival in the bone marrow.52 Specifically, these investigators found that by immunizing eosinophil-deficient mice (PHIL or ΔdblGATA1) or mice depleted of eosinophils by using anti-SiglecF antibodies resulted in a reduced number of antigen-specific plasma B cells in the bone marrow of these mice. Eosinophils were demonstrated to reside near marrow plasma B cells and produce APRIL and IL-6, suggesting eosinophils were contributory to plasma B-cell survival through these cytokines. Moreover, in a second paper, Chu et al demonstrated that eosinophils may augment secondary immunization of plasma B cells enhancing their long-term antibody production.53 Eosinophil roles in bone marrow may also include remodeling events, such as bone regeneration through release of IL-4 and IL-6.16 Currently, these investigations have been confined to a limited number of models of long-term humoral memory responses in mice and await further studies in patients. In addition, significant perturbations in short-term humoral immune responses have not been reported in eosinophil-less strains of mice and again the relationship between eosinophils and plasma B cells in humans remains unclear. However, if such a link were established in patients, these observations would have potentially significant implications for many diseases that result from abnormal expression of antibodies by plasma B cells (eg, autoimmune diseases or multiple myeloma).
Thymic development and T-cell selection
Eosinophils were first described to localize to the thymus in the 1970s54 and since have been characterized as increasing to a maximal level within 2 weeks of age in mice55 and 2-3 years in humans.56 The presence of eosinophils within the corticomedullary and medullary region have suggested that these cells may participate directly in the selection of T cells or may aid in the scavenging of dead cells that fail negative selection. Throsby et al characterized these mouse-derived eosinophils as CD11cint, CD11bhi, CD44hi cells that express TGF-β, IL-4, and IL-13 and are capable of acute class 1-dependent negative selection of T cells.55 In a recent study, Moqbel et al demonstrated in surgically removed thymus of neonates and children that eosinophils express IDO, IL-4, and IL-13; this expression was also shown to decrease with age.56 They suggest that the IDO-positive eosinophils contribute to the Th2 character of the developing thymus in normal humans by inducing apoptosis of Th1 cells through depletion of tryptophan. An alternative function of eosinophils in the thymus was demonstrated recently by Kim et al that suggests eosinophils aid macrophages in the phagocytosis of apoptotic thymocytes induced by γ-irradiation.57 Despite this circumstantial evidence linking eosinophils and the thymus, it is interesting that pathologies that would be linked with T-cell selection in vivo (eg, systemic autoimmune disease) have not been reported in eosinophil-less strains of mice. Thus, the suggestion that eosinophils have a functional role in T-cell selection in the thymus of both humans and mice remains a provocative yet still unproven hypothesis.
Metabolic syndrome: adipose, insulin, and inflammation
Metabolic syndrome is characterized by an increased risk of atherosclerosis, stroke, and type 2 diabetes. Defining the regulation of inflammatory pathways and metabolic signals is a complex problem to undertake, yet individual findings in clinical studies58 and use of animal models59 have suggested a potential role for eosinophils in maintaining metabolic homeostasis. In particular, studies in mice have highlighted a role for eosinophils59 in modulating adipocyte tissue macrophages, which are important in metabolic homeostasis.60 Specifically, Wu et al demonstrated that resident eosinophils in adipose tissue appear to mediate macrophage differentiation to the M2 phenotype, increase M2 cell numbers in fat, and are required for glucose homeostasis (ie, normal insulin sensitivity).59 Furthermore, they demonstrated that eosinophil numbers in fat tissue correlate inversely with fat deposition in animal models, suggesting that the presence of eosinophils is protective toward development of type 2 diabetes. Collectively, these studies hypothesize that eosinophils resident to adipose tissue aid in the polarization of the immune microenvironment (eg, differentiation of macrophages to the M2 phenotype) needed to sustain glucose and insulin homeostasis. These new findings for eosinophil function in mouse glucose homeostasis have yet to be examined in otherwise normal patients and patients with metabolic syndrome.
Female reproductive system
Physiologic functions of the mammalian female reproductive tract are regulated, in part, by remodeling events induced by resident and infiltrating leukocytes, suggesting a functional role for inflammatory-like processes.61 Eosinophils are found in both the developing ovary and increased in numbers in preovulatory follicles, possibly via eotaxin- or RANTES-dependent recruitment.62 The potential role of eosinophils in the ovary is purely speculative at this point; however, the demonstration that eosinophils are robust sources of the same remodeling factors noted to be required during the transition from follicle to luteal phase is highly suggestive.
Pregnancy.
Eosinophils have been shown to be a significant cellular infiltrate of the placenta and uterus,63 including the infiltration and degranulation of eosinophils in cervix of pregnant humans.64 Timmons et al have suggested that the increased presence of eosinophils in the cervix permits dilation for birth and postpartum remodeling.65 The observation that eosinophil infiltration of the cervix is also associated in time and location with the increased presence of activated macrophages65 suggests that eosinophils may contribute not only to the remodeling of the cervix directly, but may do so indirectly through the polarization of macrophages in the cervix.
Endometriosis.
During mammalian estrus, uterine eosinophils are normally absent during the menstrual cycle except during menstruation.66 Menstruation is an acute inflammatory response, yet chronic inflammation (particularly when it occurs at ectopic sites) results in endometriosis and is a prominent cause of female infertility. Interestingly, observations showing a concordance of eosinophil infiltration/eosinophil degranulation with fibrotic regions of endometriotic tissue67 suggest a potential role for eosinophils in the remodeling and fibrosis that occurs in this inflammatory disease.
Mammary gland development
Eosinophils are associated with terminal end buds of the developing mammary gland and appear to be a required component of terminal end budding and ductal branch morphogenesis. Specifically, Gouon-Evans et al demonstrated that macrophages are essential for the development of terminal end buds during development in mice and that eosinophil recruitment depends on the expression of eotaxin-1.68 The importance of these eosinophil-mediated remodeling events in the mammary gland development were foreshadowed by our observations of decreased litter size and weanling survival in IL-5–deficient nursing dams.69 Alternatively, excessive numbers of eosinophils, such as in hypereosinophilic mice that express IL-5 from T cells, also demonstrated attenuated mammary bud development,70 in this case, presumably because the inflammatory infiltration of eosinophils to the mammary tissue disrupting homeostatic development. The unresolved question is the translational character of these observations to humans.
Responses to respiratory viral infection
Respiratory viruses have become a burgeoning area of research in the pulmonary field because of their suspected causative effect(s) on asthma development and exacerbations.71 The link between eosinophils, respiratory viruses, and asthma appears to be complex but several observations are noteworthy suggesting a commonality of the role(s) of eosinophils in both humans and mice in response to viral infections: (1) Formalin-inactivated respiratory syncytial virus vaccine provided to children in the 1960s led to a hypersensitivity response that induced eosinophilia.72 In animal models, viral exposure after vaccination was demonstrated to lead to what was described as a Th2 cytokine bias with an associated eosinophilia, although the role of these eosinophils remains controversial.73 (2) Respiratory viral infections in the first few years of life are often associated with an increased incidence of asthma and a correspondingly induced pulmonary eosinophilia. Yet specific mechanisms remain the subject of debate.71 Moreover, studies have suggested a number of immune pathways linking eosinophils, viral induced-asthma, and asthma exacerbations, but these remain to be clarified in both patients and in mouse models of respiratory infection.74,75 (3) Ex vivo studies using purified proteins in both viral infection studies in culture and several in vivo animal studies of viral infection have mechanistically linked antiviral eosinophil activities to the release of secondary granule proteins. These include the demonstration that eosinophil-associated ribonucleases displayed antiviral activities toward single-stranded RNA viruses,76 the observations that major basic protein77 and eosinophil peroxidase (EPX)78 each appear to provide unique antiviral activities in mouse models of viral infection, and eosinophil activation may be linked with the binding of viral ligands to Toll-like receptors on eosinophils.79
In summary, these studies suggest that eosinophils in both humans and mice may have a potential contributory role(s) to the immune responses associated with viral vaccination/infection as either a fundamental innate host response to viral infection, a component of acquired immune responses to viral infection, and/or a contributor to host remodeling/repair required in the recovery phase post-infection.
Pulmonary hypertension
Pulmonary hypertension is characterized by the restriction of arterial blood vessels within the lungs, leading to impaired oxygen exchange. Weng et al used a novel mouse model that is deficient in a cytokine secreted by adipose tissue, adiponectin, to show a unique connection between pulmonary hypertension and eosinophils.80 Specifically, airways allergen provocation of adiponectin-deficient mice (adn−/−) that were also eosinophil-deficient resulted in attenuated pulmonary hypertension as well as decreased remodeling of the lung vasculature.80 More importantly, they were also able to demonstrate directly that eosinophil granule products are able to induce arterial smooth muscle proliferation in vitro, suggesting eosinophils are essential for the vasculature remodeling that leads to pulmonary hypertension. The hypothesis proposed is that eosinophils release angiogenic and vascular remodeling mediators, such as vascular endothelial growth factor and TGF-β, which increases extracellular matrix remodeling and vascular inflammation. Significantly, Puxeddu et al have also demonstrated that eosinophils have the capacity for endothelial remodeling.81 The association of eosinophils and pulmonary hypertension thus far is limited to mouse-based studies. Nonetheless, these studies are suggestive that a similar association may represent and underappreciated pathway occurring in human subjects.
Acute lung injury
Acute lung injury are related pulmonary conditions associated with significant lung histopathologic changes ultimately resulting in an inability to exchange oxygen sufficiently, increasing patient risk of mortality and morbidity. These diseases are often idiopathic and linked with a multitude of factors, including exposure to toxic chemicals, infection/sepsis, and complications of other diseases. Although acute lung injury is generally not thought of as an eosinophilic disease,82 Willetts et al demonstrated that increased numbers of eosinophils are associated with survival of acute lung injury patients, suggesting a potential diagnostic value of assessing for eosinophils and their associated activities in these patients.83
Transplant rejection
Transplant rejection (ie, GVHD) is an acute or chronic inflammatory/remodeling phenomenon that ultimately results in destruction of the transplanted cells/tissue and increased mortality and morbidity. Significantly, intact and degranulated eosinophils have been demonstrated to localize to rejected human donor tissue in a variety of organs (eg, kidney84 and heart85 ). Currently, the role of eosinophils in these cases has primarily been limited to that of a marker for acute GVHD without a thorough understanding of the role that eosinophils may play. Several possibilities exist, including that the eosinophil infiltrate is an inflammatory “consequence” of the complex immunobiology surrounding host responses to the transplant or that eosinophils are immune cells actively maintaining homeostatic transplant integrity. Alegre et al suggested multiple immune mechanisms of GVHD whereby eosinophils predominate as the mediators of Th2-induced rejection.86 For example, studies have demonstrated that in Th1/IL-17–deficient mouse models of organ transplant,87 eosinophil infiltration into the tissue was a predominant feature. Moreover, the absence of IL-5 or eosinophils (antibody depletion) attenuated transplant rejection in MHC-incompatible allograft transplant models.88,89 The novelty of these observations is again that eosinophils may promote and/or amplify Th2 immune responses associated with organ/tissue rejection. In addition, depletion of eosinophils in mice (administration of antibody to IL-5) that develop chronic GVHD after cardiac transplant led to reduced collagen and elastin deposition,88 suggesting a direct role for eosinophils in the fibrotic processes (ie, remodeling/repair) linked with transplant rejection. Thus, the ability of eosinophils to engage in remodeling/repair and to modulate the immune microenvironment supports a suggested paradigm in which these granulocytes contribute to post-transplant organ homeostasis.
IBD
Inflammatory bowel diseases (IBD), such as Crohn disease and ulcerative colitis, are characterized by significant inflammation and tissue remodeling in the small intestine and colon, respectively, resulting in diarrhea, constipation, and weight loss in patients.90 Although eosinophils are found in the gastrointestinal tract at baseline (stomach to rectum91 ), their numbers and their degranulation are dramatically increased in IBD.92 Mouse models of IBD have also provided important insights showing that eosinophils are potential contributors to the inflammation and remodeling that occur in these diseases. For example, disease progression in spontaneous mutant mice that mimic IBD disease in humans (ie, SAMP mutations) are attenuated by depletion of eosinophils (administration of IL-593 or chemokine receptor 394 antibody). It is once again noteworthy that in these mouse models of IBD, depletion of eosinophils also resulted in a reduction in the number and activation of effector CD4+ Th2 T cells in the draining lymph nodes and their ability to be activated on stimulation.93 Similarly mice deficient in IL-5,95 EPX,95 and eosinophils96 are partially protected from chemical-induced models of colitis. We would suggest that the remarkably complex immunity of the gut occurring as a consequence of the bacterial burden of this compartment (and the necessary containment it requires of the host) may explain why uniquely the gastrointestinal tract has a rather robust resident eosinophil population. Interestingly, although the primary function of these eosinophils may be immunoregulative in character, recent observations of unique antibacterial activities97,98 may be equally underappreciated effector functions of these gut-associated granulocytes.
Duchenne muscular dystrophy
Duchenne muscular dystrophy is a genetic mutation in dystrophin that leads to mechanical injury of the muscle tissue over time followed by the infiltration of activated leukocytes. The ensuing inflammation has been proposed to lead to tissue fibrosis and mechanical failure of the muscle often leading to death as a consequence of cardiac failure. A key (yet largely unexplained) feature of Duchenne muscular dystrophy is the eosinophil accumulation occurring in the injured muscle and its correlation with disease severity.99 Significantly, Wehling-Henricks et al have demonstrated, using a mouse model of Duchenne muscular dystrophy (ie, dmx−/−), that eosinophils appear to contribute to the disease process through the deposition of major basic protein, which resulted in both site-specific fibrosis and induced changes in inflammatory immune responses leading to disease-specific muscle pathologies.100
Eosinophil-nerve interactions leading to twitch and itch
Eosinophil-nerve interactions have been documented in the literature and represent a collection of studies that suggest an underlying importance as part of the potential remodeling/immune events contributing to tissue-nerve homeostasis. Two areas of translational research investigating the potential clinical implications of eosinophil-nerve interactions are worth noting as they highlight the complexity of these events as well as their possible significance in health and disease. In the first area, Costello et al have demonstrated that eosinophils/eosinophil granule proteins in both human biopsies,101 and guinea pig models of allergic respiratory inflammation102 are often found in proximity of parasympathetic nerves and may lead to an inhibition of acetylcholine binding as part of a negative feedback loop modulating airway smooth muscle tone.103 Localization of eosinophils to these parasympathetic neurons may be through eotaxin released by neurons,104 and subsequent eosinophil-nerve interactions may be direct in character as eosinophils have been shown to express classic neuromediators, such as neuropeptides and neurotrophins as well as their receptors.15 In the second area of research regarding eosinophil-nerve interactions, Foster et al have demonstrated that degranulating eosinophils in humans colocalize with nerves of the dermis and increased with the severity of contact dermatitis.105 Studies with eosinophil-less PHIL mice and mouse models of contact dermatitis have recently confirmed a link among eosinophils, nerve growth and branching, and increased levels of itch response (J.J.L., unpublished observations, July 2012). Collectively, these observations suggest eosinophil-nerve interactions first identified in the lung may also exist in other tissue compartments with consequences to nerve function in other tissues.
Multiple sclerosis and neuromyelitis optica
Multiple sclerosis and neuromyelitis optica are diseases of demyelination of the CNS with progressive or remitting/relapsing occurrence. Multiple sclerosis primarily involves the brain and spinal cord and is considered a Th1/Th17 autoimmune disease. Mouse models of multiple sclerosis (experimental allergic encephalomyelitis) generated by immunizing animals with myelin protein peptides also have demonstrated a role (although largely undefined) for Th2 cytokines (eg, IL-4106 and IL-5107 ), and possibly eosinophils in disease suppression. Neuromyelitis optica is a similar disease now thought to be an autoimmune reaction to aquaporin-4 targeting the spinal cord and optic nerve. Significantly, eosinophils are elevated in the optic lesions of patients with neuromyelitis optica, and CSF displays increased Th2 cytokines and the presence of eosinophil granule proteins.108,109
Cancer and tumor biology
Regardless of the diverse etiology of cancers, eosinophils are a common cellular infiltrate found in nearly all solid tumors and cancers of epithelial origin. Examples of eosinophil-containing cancers in humans3 include, and are not limited to gastrointestinal, uterine, cervical, mammary, bladder, glioblastoma, pancreatic, and oral.3,110 Although eosinophil infiltration of tumors is common, the cause and consequences (ie, protumorigenic vs antitumorigenic) of this recruitment and accumulation are unclear. Recent studies in mouse models of cancer and patient biopsies have suggested 2 somewhat independent mechanisms potentially linking eosinophils and cancer: (1) The recruitment of eosinophils is probably a host inflammatory response to the tumor as a consequence of cell death/necrosis and/or hypoxia that elicits the release of danger-associated molecular patterns.111 This perspective suggests that eosinophils are a component of innate host defense to a perceived threat, leading to remodeling/repair that may increase or decrease tumor growth. (2) Tumor infiltrating eosinophils are part of host immune responses that include abilities to polarize T-cell functions, modulate humoral responses, or even be immunosuppressive in character. For example, the presence of IDO-positive eosinophils,112 release of eosinophil granule proteins,113 and the presence of IL-5114 have all been shown to be protumorigenic, whereas in other instances eosinophils are antitumorigenic.115 Indeed, animal studies addressing the relative role of eosinophils have been confounding.115,116 Regardless of the specific role(s) eosinophils have in cancer, these eosinophil-mediated activities (either remodeling/repair or immunoregulatory) are clearly not bystander effects, and these cells probably contribute both potentially significant protumorigenic and/or antitumorigenic activities.
Eosinophils: biomarkers in translational strategies of patient care
The classic example of eosinophils as a biomarker for disease and severity is in the diagnosis of asthma and asthma exacerbations. In this example, elevated levels of eosinophils in sputum (as detected by hematoxylin and eosin-stained differentials) correlate with disease severity and can be used in the management of patient care.33 However, novel assays with the ability to identify and quantify eosinophil activities are now beginning to show promise as diagnostic tools. For example, we have demonstrated, through the use of a novel anti-eosinophil peroxidase monoclonal antibody (EPX-mAb), that this detection method was capable of identifying tissue infiltrating eosinophils in patient biopsies, as well as evidence of degranulation, that was significantly beyond the ability to do so through conventional evaluations of hematoxylin and eosin-stained slides.83,117 In addition, biochemical assays of eosinophil activities118 and eosinophil granule proteins have been used as novel assays to link eosinophil activities to disease severity and/or patient outcomes as is true of unique ELISA assays of eosinophil granule proteins (eg, EPX119 ). The availability of these novel methodologies is of particular interest moving forward as the roles of eosinophils expand from the “classic” eosinophil-associated diseases of asthma and helminth infection to the myriad of diseases and pathologies linked with eosinophils.
In conclusion, remarkably, over the past 10 years, the list of eosinophil-associated diseases has neither narrowed nor remained the same. Instead, this list has dramatically grown and seems to be limited only by the curiosity and cleverness of investigators caring for patients or studying particular diseases. In addition, the same can be said of more basic research studies defining eosinophil effector functions and their potential roles in the molecular and cellular processes underlying homeostasis. Thus, when asked about the roles of eosinophils in health and disease, it now seems appropriate to begin any discussion with “Did you know …?”
Acknowledgments
The authors thank persons from within Lee Laboratories not listed as authors for invaluable contributions, including Dr Sergei Ochkur, Dr Rachel Condjella, Alfred Doyle, and Dr Michael McGarry; friends within the community who for years have provided us with ideas and thoughtful reflection in our quest of trying to understand all things eosinophil; Mayo Clinic Arizona medical graphic artist Marv Ruona and Joseph Esposito of Research Library Services for their tireless efforts; and the Lee Laboratories administrative staff (Linda Mardel and Charlie Kern), without whom we could not function as an integrated group nor achieve the degree of success that we have experienced.
This work was supported by the National Institutes of Health (grants HL065228 and RR109709, J.J.L.; grant HL058723, N.A.L.), American Heart Association (11SDG7510043; E.A.J.), and Mayo Foundation for Medical Education and Research.
National Institutes of Health
Authorship
Contribution: E.A.J., J.J.L., and N.A.L. conceived, developed, and wrote the review; and R.A.H. provided essential clinical insights and perspective.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: James J. Lee, Mayo Clinic School of Medicine, Department of Biochemistry and Molecular Biology, Mayo Collaborative Research Building, Mayo Clinic Arizona, 13400 E Shea Blvd, Scottsdale, AZ 85259; e-mail: jjlee@mayo.edu; and Nancy A. Lee, Mayo Clinic School of Medicine, Department of Biochemistry and Molecular Biology, Mayo Collaborative Research Building, Mayo Clinic Arizona, 13400 E Shea Blvd, Scottsdale, AZ 85259; e-mail: nlee@mayo.edu.