Abstract
Recent evidence suggests that kindlin-3 is a major coactivator, required for most, if not all, integrin activities. Here we studied the function of kindlin-3 in regulating NK cell activation by studying a patient with kindlin-3 deficiency (leukocyte adhesion deficiency-III). We found that kindlin-3 is required for NK cell migration and adhesion under shear force. Surprisingly, we also found that kindlin-3 lowers the threshold for NK cell activation. Loss of kindlin-3 has a pronounced effect on NK cell–mediated cytotoxicity triggered by single activating receptors. In contrast, for activation through multiple receptors, kindlin-3 deficiency is overcome and target cells killed. The realization that NK cell activity is impaired, but not absent in leukocyte adhesion deficiency, may lead to the development of more efficient therapy for this rare disease.
Introduction
Integrins play fundamental roles in regulating many biologic activities.1-4 In hematopoietic cells, integrins undergo allosteric conformational changes in response to various activation signals,5 and proteins, such as talins, were demonstrated to be critical for integrin activation in all cell types investigated.6 Recent evidence suggests that kindlins, a small family of intracellular proteins (which includes 3 members), cooperate with talin, through binding to distinct motifs located in the short tails of the β-integrin subunits, and translate the signals derived from the integrins into a functional outcome.7-10 Of the 3 known kindlin proteins, kindlin-3 is confined to hematopoietic cells.11
The importance of kindlin-3 in integrin activity was demonstrated most noticeably through the recent identification of patients with leukocyte adhesion deficiency (LAD) type III.12-18 These patients have recurrent clinical bleeding and infections few days after birth.13-19 The disease is very rare, and only a few cases were described so far.16,18,20 Studies performed on platelets, lymphocytes, and PMNs derived from patients demonstrated that loss of kindlin-3 affects integrin activation in all cell types studied.13-19
Natural killer (NK) cells kill virus-infected cells, bacteria, and tumor cells without prior activation,21 although recent data demonstrated that NK cells could possess a certain type of memory.22,23 NK cell activity results from a balance between activating and inhibitory signals.24 Killing of target cells by NK cells is a multistep process. The first step is binding of NK cells to target cells, which is mediated by adhesion molecules and by the interaction of NK cell receptors with their ligands. This step is followed by generation of an activating NK synapse, translocation of cytotoxic granules,25 and release of perforin and granzymes toward the target cells.26
The leukocyte integrin LFA-1 (αLβ2 integrin, CD11a/CD18) plays an essential role in many aspects of NK cell behavior. Importantly, LFA-1 acts beyond merely regulating adhesion because signaling through LFA-1 in NK cells is also directly involved in regulating NK cell cytotoxicity.27,28
Here we examined the function of NK cells derived from a LAD-III patient presenting an autosomal recessive stop codon mutation in kindlin-3.15 We demonstrate that kindlin-3 influences the threshold at which NK cells are activated and that absence of kindlin-3 affects some, but not all, NK cell activities.
Methods
LAD-III patient
NK cells were obtained from a female baby carrying an autosomal recessive stop codon in kindlin-3 (nucleotide 687). The patient was described previously.15 The work was carried out over a period of 2 years (patient age, 1-3 years) and NK cells were obtained at various time points during this period. The Institutional Review Board of Haddasha Hospital has approved this work, which was conducted in accordance with the Declaration of Helsinki.
Cells and reagents
NK cells were isolated and grown as described previously.29 NK cells were obtained from the LAD-III patient at various time points (1-3 years of age) and in parallel, from various healthy controls of different ages and genders. NK cells derived from the control and the patient were treated under the same conditions. The production and purification of the NKp30-Ig, NKp44-Ig, and the NKp46-Ig fusion proteins were performed as previously described.30
NK cell activation of coated slides
Glass chamber slides were coated with mAb or recombinant proteins in PBS and blocked with complete culture medium. NK cells (5 × 105 cells/mL in 25mM HEPES in complete medium) were incubated on slides for 6 minutes at 37°C, fixed with 4% paraformaldehyde, and F-actin visualized by staining with phalloidin conjugated to ATTO-647N (Attotech). Bright-field, IRM, and fluorescence images were captured at the slide surface. The area of cell spreading and distance to the cell perimeter from the centroid of the contact with the slide along 360 radii were measured as described previously.31
Migration
NK cells in complete media were treated with 5mM MgCl2 and 1mM EGTA or left untreated. Cells were allowed to settle on ICAM-1–coated chamber slides for 10 minutes, and then nonattached cells were removed by gentle washing. Bright-field and IRM images were captured at the slide surface every 5 seconds between 10 and 30 minutes by confocal microscopy (Leica SP5 RS) using a 63× water immersion lens (NA 1.2). Cell speeds were quantified in Image J using the manual tracking tool.
Laminar flow adhesion assay
Adhesion assays were performed on ICAM-Fc overlaid on precoated protein A polystyrene plates as described.15 Substrate–coated polystyrene plates were each assembled on the lower wall of a standard flow chamber (260-μm gap), as described. Cells were washed with H/H medium, resuspended in binding medium, and perfused through the flow chamber. Rapid development of LFA-1 adhesion strengthening and resistance to shear forces was determined by allowing NK cells to settle onto the substrate for 1 minute at stasis. Flow was then initiated and increased step-wise every 5 seconds by a programmed set of rates. At the indicated shear stresses, the number of cells that remained bound was monitored by live videomicroscopy and expressed relative to the number of cells originally settled on the substrate.
Cytotoxicity and redirected assays
Degranulation assay
CD107a lysosome-associated membrane protein-1 (LAMP-1) expression was used to measure NK-cell degranulation. NK cells were incubated with or without 1106mel target cells at an E/T ratio of 1:1 for 2.5 hours. The biotinylated anti-CD107a (BD Biosciences PharMingen) was added directly to the assay. Cells were then stained with PE-conjugated anti-CD56 (BD Biosciences PharMingen) and with Cy5-conjugated sreptavidin (Jackson Immunoresearch Laboratories) and analyzed by FACS.
Quantitative RT-PCR
The following primers were used to amplify the kindlin3 gene: 5′ kindlin3 AGCAGATCAATCGCAAGCAG and 3′ kindlin3 ATCCCGTACTTGTCCAGTGT. DNA was amplified on an ABI PRISM 7500 Real-Time PCR System (Applied Biosystems). The relative amount of the kindlin3 mRNA was normalized to the level of GAPDH and HPRT mRNA level.
Statistical analysis
For statistical comparison between groups, the paired 2-tailed Student t test was used. Analyses were performed using the statistics tool of Microsoft Excel. Statistical analysis of cell spreading and cell migration was carried out using GraphPad software (Prism). Mean values are shown unless otherwise indicated. Errors and error bars represent SEM unless otherwise stated. In statistical analysis, for 2 sample groups, data were analyzed by paired t test. For comparing more than 2 sample groups, data were analyzed by 1-way ANOVA. For parametric data, a Bonferroni posttest was applied; and for nonparametric data, a Kruskal-Wallis test was carried out followed by Dunn multiple comparison posttest.
Results
NK cell characterization of a LAD-III patient
To test the function of kindlin-3 in human NK cells, we characterized the NK cell phenotype in a female baby with LAD-III deficiency. This patient is a sibling of a previously reported LAD-III patient33 who died of the disease (Figure 1A) and of another unreported sibling who died prenatally. The patient was recently reported to carry an autosomal recessive stop codon in kindlin-3, and no kindlin-3 could be detected in her blood.15 We initially verified by quantitative RT-PCR that human NK cells indeed express kindlin-3 and that the patient is deficient for kindlin-3 expression (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). We also determined that NK cells percentages of the LAD-III patient are similar to that of healthy controls (data not shown). In contrast, NK cell (as well as other lymphocytes) numbers in the blood of the LAD-III patient were 10 times higher than healthy controls (data not shown). Importantly, throughout this work, NK cells were obtained from the patient over a period of 2 years (age 1-3 years) and various healthy controls (ages 20-45 years) were used.
Profile of NK cells derived from a LAD-III patient. (A) Family pedigree of the LAD-III patient. Striped symbols represent the heterozygous parents; full gray symbols, LAD-III patients; and the X symbol, the sibling who unfortunately died. (B) Freshly derived (2 left columns) and IL-2–activated (2 right columns) NK cells were obtained from the LAD-III patient and from a healthy control (indicated above the histograms). NK cells were stained for the expression of various activating receptors (indicated at the left part of the figure). Numbers in the figure indicates median fluorescence intensity of the gray histograms. (C) NK cells from the LAD-III patient and from a healthy control (indicated in the left part of the figure) were stained for the expression of LFA-1. (B-C) Black empty histogram represents the staining of the cells with a control mAb; gray empty histogram, specific staining. Figure shows 1 representative experiment of 3 performed.
Profile of NK cells derived from a LAD-III patient. (A) Family pedigree of the LAD-III patient. Striped symbols represent the heterozygous parents; full gray symbols, LAD-III patients; and the X symbol, the sibling who unfortunately died. (B) Freshly derived (2 left columns) and IL-2–activated (2 right columns) NK cells were obtained from the LAD-III patient and from a healthy control (indicated above the histograms). NK cells were stained for the expression of various activating receptors (indicated at the left part of the figure). Numbers in the figure indicates median fluorescence intensity of the gray histograms. (C) NK cells from the LAD-III patient and from a healthy control (indicated in the left part of the figure) were stained for the expression of LFA-1. (B-C) Black empty histogram represents the staining of the cells with a control mAb; gray empty histogram, specific staining. Figure shows 1 representative experiment of 3 performed.
We tested the expression of various NK cell receptors on freshly derived and IL-2–activated NK cells obtained from the patient and compared them with various healthy controls (Figure 1B-C; supplemental Figures 2 and 3). In a representative healthy control (shown in Figure 1), small differences in the level of expression of various NK cell receptors were observed, but these were not consistent and in the range of variation found between different healthy donors (Figure 1B; supplemental Figures 2 and 3). Furthermore, NKp44 expression was up-regulated on the IL-2 activated LAD-III patient and on healthy control NK cells, to a similar extent (Figure 1B), suggesting that, even after activation, the expression of surface proteins on the LAD-III patient cells seemed to be normal. Because kindlin-3–deficiency affects integrin activation, we also compared the expression of LFA-1 (the major integrin expressed by NK cells) on LAD-III NK cells with that of healthy controls and observed no major difference (Figure 1C; and data not shown). Thus, we conclude that the NK cell receptor repertoire of the patient is not substantially different from other healthy donors.
Kindlin-3 is important for the activation of NK cells by single NK cell receptor
Because of ethical reasons concerning the patient's very young age, which allowed the use of minimal amounts of blood samples, we performed all subsequent experiments using IL-2–activated NK cells that were grown in culture (purity > 99%, data not shown).
We initially tested the role of kindlin-3 in NK cell cytotoxicity by stimulating a range of different activating receptors independently, as indicated in Figure 2A. We noticed that cytotoxicity of the LAD-III patient NK cells was impaired for all activating receptors tested (Figure 2A). Similar results were obtained across assays done at different time points as well as when different healthy donors were used (summarized in supplemental Figure 4A).
Impaired LAD-III NK cell cytotoxicity on triggering of a single receptor. (A) P815 cells were incubated either with no mAb or with various mAbs (indicated on the x-axis of the figure) to stimulate redirected NK cell cytotoxicity. The E:T ratio was 3:1. Gray columns represent the healthy NK cell cytotoxicity; and white columns, the cytotoxicity of the LAD-III NK cells. Data are mean ± SD. Error bars (SD) are derived from triplicates. **P < .005 (2-tailed t test). Shown is 1 representative experiment of 5 performed. (B) FACS analysis of JEG3 cells transfected to express various NKG2D ligands (left panels). The same cells were also stained for the expression of ICAM-1 (right panels). The gray empty histogram represents the staining of the cells with a control mAb; and the black empty histogram, the specific staining. Figure shows 1 representative experiment of 3 performed. (C) The JEG3 parental cells and the various transfectants were tested for killing by bulk NK cell cultures derived from a healthy control (gray columns) or by NK cells derived from the LAD-III patient (white columns). The E:T ratio was 5:1. Figure shows 1 representative experiment of 2 performed. Data are mean ± SD. Error bars (SD) are derived from triplicates. *P < .05 (2-tailed t test). **P < .005 (2-tailed t test). NS indicates not significant. (D) NK cells derived from a healthy donor or from the LAD-III patient were assayed for their ability to kill the B-cell line Daudi or Daudi coated with anti-CD20 (1 μg/mL). Data are mean ± SD. Error bars were derived from triplicates. Shown is 1 experiment of 3 performed.
Impaired LAD-III NK cell cytotoxicity on triggering of a single receptor. (A) P815 cells were incubated either with no mAb or with various mAbs (indicated on the x-axis of the figure) to stimulate redirected NK cell cytotoxicity. The E:T ratio was 3:1. Gray columns represent the healthy NK cell cytotoxicity; and white columns, the cytotoxicity of the LAD-III NK cells. Data are mean ± SD. Error bars (SD) are derived from triplicates. **P < .005 (2-tailed t test). Shown is 1 representative experiment of 5 performed. (B) FACS analysis of JEG3 cells transfected to express various NKG2D ligands (left panels). The same cells were also stained for the expression of ICAM-1 (right panels). The gray empty histogram represents the staining of the cells with a control mAb; and the black empty histogram, the specific staining. Figure shows 1 representative experiment of 3 performed. (C) The JEG3 parental cells and the various transfectants were tested for killing by bulk NK cell cultures derived from a healthy control (gray columns) or by NK cells derived from the LAD-III patient (white columns). The E:T ratio was 5:1. Figure shows 1 representative experiment of 2 performed. Data are mean ± SD. Error bars (SD) are derived from triplicates. *P < .05 (2-tailed t test). **P < .005 (2-tailed t test). NS indicates not significant. (D) NK cells derived from a healthy donor or from the LAD-III patient were assayed for their ability to kill the B-cell line Daudi or Daudi coated with anti-CD20 (1 μg/mL). Data are mean ± SD. Error bars were derived from triplicates. Shown is 1 experiment of 3 performed.
Importantly, for all experiments comparing the patient with healthy donors, we first validated that the expression levels of each NK cell receptor is similar between LAD-III NK cells and different controls (for example, in the redirected experiment presented in Figure 2A the median fluorescence intensities were: NKp30-30 and 25.7, NKp44-15.5 and 19.6, NKp46-10.5 and 7.1, NKG2D-31.6 and 30.2 and CD16-139.5 and 137 for healthy and LAD-III PBLs, respectively).
We next compared direct (natural) killing of target cells through individual activating receptors. For that, we used the JEG3 cell line, which does not express any known NK cell ligands and is therefore resistant to NK cell killing.34,35 JEG3 cells were transfected to express MICA and ULBP2 (Figure 2B left columns) and verified for expression of similar levels of ICAM-1 (Figure 2B right columns). As can be seen in Figure 2C, LAD-III NK cells were impaired in their ability to kill JEG3–transfected cells. Similar results were obtained using additional healthy donors as controls and at various time points in the 2-year period of this research (summarized in supplemental Figure 4B).
Because we also observed that LAD-III NK cell cytotoxicity was impaired when engaging CD16, we examined the ability of LAD-III NK cells to mediate killing via ADCC. Cells of the B cell line, Daudi, were incubated with or without antibody against CD20 (which is expressed on Daudi cells) and then incubated with LAD-III NK cells or NK cells derived from healthy donors. Whereas NK cell killing of the Daudi cells was similar between the healthy control and the patient, the ADCC activity of CD16 was impaired in the absence of kindlin-3 (Figure 2D). Similar results were obtained with additional healthy donors (summarized in supplemental Figure 4C). Thus, in the absence of kindlin-3, NK cell cytotoxicity mediated by a single NK receptor is strongly impaired.
The activation threshold for NKG2D–mediated F-actin polymerization and for symmetric spreading is altered in the LAD-III–deficient NK cells
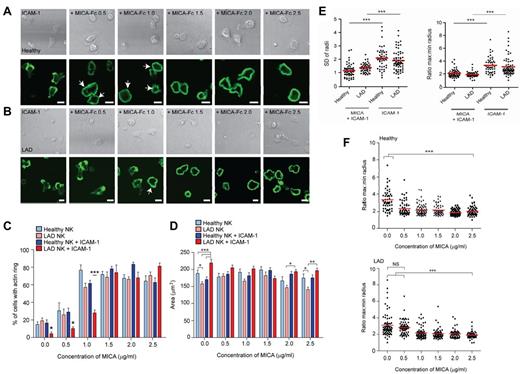
We previously reported31 that ligation of LFA-1 on NK cells induces asymmetric cell spreading and migration (also shown here in supplemental Figure 5). However, ligation of the activating receptor NKG2D results in a “stop” signal, symmetric cell spreading, and formation of a peripheral ring of filamentous actin (F-actin), even in the presence of LFA-1 costimulation (supplemental Figure 5, lower panels). Because we found that NKG2D-mediated cytotoxicity is less efficient in LAD-III patient NK cells, we next investigated the ability of LAD-III NK cells to respond to NKG2D ligation. Specifically, we compared the extent to which healthy and LAD-III NK cells stimulated with increasing concentrations of the NKG2D ligand, MICA, and ICAM-1, were able to form polymerized rings of F-actin at the synapse periphery. In these experiments, rings of actin filaments are scored as a synapse, whereas an interrupted or patchy ring represents a kinapse, that is, the cell retains motility and spreads assymetrically.31 Peripheral rings of F-actin could be detected much more frequently for healthy NK cells stimulated on ICAM-1 plus 0.5 μg/mL or 1.0 μg/mL MICA-Fc at 28% ± 3.3% and 62% ± 3.5%, respectively (Figure 3A), compared with LAD-III NK cells for which only 10% ± 1.9% and 29% ± 4% of cells formed F-actin rings, respectively (Figure 3B). At higher concentrations of MICA-Fc (1.5-2.5 μg/mL), a similar level of activation was observed for all conditions tested with > 60% of cells forming peripheral F-actin rings (Figure 3A-B). The difference in the frequency of NK cells forming an F-actin ring was consistent for various healthy donors (supplemental Figure 6), indicating that the substantially lower frequency of F-actin ring formation is particular to the patient's cells.
The activation threshold for NKG2D-mediated F-actin polymerization and for symmetric spreading is altered in LAD-III NK cells. (A) Representative bright-field (top panels) and fluorescence images of F-actin (bottom panels) for healthy NK cells stimulated on surfaces coated with ICAM-1 (2.5 μg/mL) with increasing concentrations of MICA-Fc (0-2.5 μg/mL). (B) Representative bright-field (top panels) and fluorescence images of F-actin (bottom panels) for LAD-III NK cells stimulated as in panel A. White arrows indicate activated cells. Bars represent 10 μm. (C) The proportion of healthy or LAD-III NK cells forming a peripheral ring of F-actin in response to surfaces coated with poly lysine and indicated concentrations of MICA-Fc (light blue and pink, respectively) or surfaces coated with ICAM-1 and indicated concentrations of MICA-Fc (blue and red, respectively). Data are mean ± SEM from 4 independent experiments; n = 50-90. ***P < .0005 (1-way ANOVA). *P < .05 (1-way ANOVA). (A-C) A complete ring of actin filaments is scored as a synapse, whereas an interrupted or patchy ring represents a kinapse. (D) The area of cells attached to the slide surface, as assessed by IRM imaging, was measured for healthy NK cells and LAD-III NK cells on surfaces coated with ICAM-1 and increasing concentrations of MICA-Fc (blue and red, respectively) and for healthy and LAD-III NK cells on surfaces coated with poly lysine and increasing concentrations of MICA-Fc (light blue and pink, respectively). Data are mean ± SEM from 4 independent experiments; n = 50. ***P < .0005 (1-way ANOVA). **P < .005 (1-way ANOVA). *P < .05 (1-way ANOVA). (E) The distance from the cell centroid of phalloidin-AlexaFluor488-stained healthy or LAD-III NK cells to the circumference was measured at 360 radii. The symmetry of the cells stimulated on ICAM-1–coated slides with or without MICA-Fc (2.5 μg/mL) is represented as the SD of the radial distances and the ratio of maximum and minimum lengths; n = 50-90. ***P < .0005. (F) The ratio of maximum and minimum lengths for healthy or LAD-III NK cells stimulated on surfaces coated with ICAM-1 and indicated concentrations of MICA-Fc; n = 50-90. *P < .05. NS indicates not significant.
The activation threshold for NKG2D-mediated F-actin polymerization and for symmetric spreading is altered in LAD-III NK cells. (A) Representative bright-field (top panels) and fluorescence images of F-actin (bottom panels) for healthy NK cells stimulated on surfaces coated with ICAM-1 (2.5 μg/mL) with increasing concentrations of MICA-Fc (0-2.5 μg/mL). (B) Representative bright-field (top panels) and fluorescence images of F-actin (bottom panels) for LAD-III NK cells stimulated as in panel A. White arrows indicate activated cells. Bars represent 10 μm. (C) The proportion of healthy or LAD-III NK cells forming a peripheral ring of F-actin in response to surfaces coated with poly lysine and indicated concentrations of MICA-Fc (light blue and pink, respectively) or surfaces coated with ICAM-1 and indicated concentrations of MICA-Fc (blue and red, respectively). Data are mean ± SEM from 4 independent experiments; n = 50-90. ***P < .0005 (1-way ANOVA). *P < .05 (1-way ANOVA). (A-C) A complete ring of actin filaments is scored as a synapse, whereas an interrupted or patchy ring represents a kinapse. (D) The area of cells attached to the slide surface, as assessed by IRM imaging, was measured for healthy NK cells and LAD-III NK cells on surfaces coated with ICAM-1 and increasing concentrations of MICA-Fc (blue and red, respectively) and for healthy and LAD-III NK cells on surfaces coated with poly lysine and increasing concentrations of MICA-Fc (light blue and pink, respectively). Data are mean ± SEM from 4 independent experiments; n = 50. ***P < .0005 (1-way ANOVA). **P < .005 (1-way ANOVA). *P < .05 (1-way ANOVA). (E) The distance from the cell centroid of phalloidin-AlexaFluor488-stained healthy or LAD-III NK cells to the circumference was measured at 360 radii. The symmetry of the cells stimulated on ICAM-1–coated slides with or without MICA-Fc (2.5 μg/mL) is represented as the SD of the radial distances and the ratio of maximum and minimum lengths; n = 50-90. ***P < .0005. (F) The ratio of maximum and minimum lengths for healthy or LAD-III NK cells stimulated on surfaces coated with ICAM-1 and indicated concentrations of MICA-Fc; n = 50-90. *P < .05. NS indicates not significant.
These data suggest that LAD-III NK cells may require a stronger activating signal to override the migratory signals following stimulation of LFA-1. Indeed, in the absence of ICAM-1, increasing concentrations of MICA resulted in similar activation of healthy and LAD-III NK cells (Figure 3C). In contrast, when ICAM-1 was present, which allows NK cells to migrate, a significantly higher concentration of MICA was required to stop and activate LAD-III NK cells compared with healthy NK cells (Figure 3C). Thus, the threshold needed for the NKG2D-mediated “stop” signal in the presence of signals from LFA-1 is higher in the absence of kindlin-3.
To test whether or not there was also a change in the threshold at which LAD-III NK cells were activated to form a symmetric synapse, we also characterized the morphology of cells. We have previously reported that the area of attachment of healthy NK cells stimulated through LFA-1, NKG2D, or both LFA-1 and NKG2D is similar, but the degree of cell symmetry is dramatically different.31 Quantification of the area of attachment of LAD-III NK cells was similar to that of healthy NK cells when stimulated on slides coated with MICA-Fc (Figure 3D).
On surfaces coated with ICAM-1 alone (ie, 0 MICA on graph), LAD-III NK cells had a significantly increased cell surface contact area (219 ± 7.8 μm2) compared with healthy controls (170 ± 7.6 μm2). This was consistent with observations of the LAD-III NK cells imaged on ICAM-1, which revealed that LAD-III NK cells had a normal migratory morphology (supplemental Figure 7) but that the cells appeared unable to retract their uropod and therefore extended over a larger area of the slide than healthy cells, which could migrate normally.
There were also differences in cell surface area of LAD-III and healthy NK cells at higher concentrations of MICA (2.0-2.5 μg/mL). Specifically, LAD-III NK cells formed F-actin rings at the synapse periphery to a similar frequency as healthy cells at these concentrations (Figure 3C), but the magnitude by which cells spread was reduced (Figure 3D). This suggests that kindlin-3 may also play a role in spreading in the absence of ICAM-1 co-ligation.
To analyze the differences in the symmetry of the spreading response, the distance to the cell perimeter from the centroid of the contact with the slide was measured along 360 radii in fixed cells. The SD of the radii and the ratio of maximum to minimum lengths (Figure 3E) revealed no difference between the healthy NK cells and the LAD-III NK cells. This is because both showed more symmetric morphology when spreading on MICA with ICAM-1, compared with ICAM-1 alone, as indicated by a lower SD or radii and smaller maximum/minimum radius morphology (Figure 3E). However, as presented in Figure 3C, LAD-III NK cells required a higher concentration of MICA (> 1.0 μg/mL) to spread symmetrically compared with healthy NK cells (Figure 3F).
Thus, compared with healthy NK cells, after stimulation of LFA-1, a higher threshold of MICA is required to deliver a “stop” signal to LAD-III NK cells to enable the formation of an F-actin ring and a symmetric spreading.
CD16-mediated symmetric spreading and F-actin polarization is kindlin-3–dependent
Previous reports demonstrate that the function of CD16 is very much dependent on LFA-1 and that CD16 induces strong inside-out signals for LFA-1.36 Because we had found that CD16-mediated killing was impaired in LAD-III NK cells, we examined whether kindlin-3 is required for the early activation of NK cells through CD16. We first stimulated LAD-III and healthy NK cells, through anti-NKG2D and anti-CD16, and examined the formation of the F-actin ring and the area of contact with the slide. In agreement with the results presented in Figure 3A through C, when the LAD-III NK cells, or the healthy NK cells were stimulated by anti-NKG2D mAb, cells formed a peripheral ring of F-actin (Figure 4A left panels). Similarly, healthy NK cells stimulated with anti-CD16 also formed F-actin rings (Figure 4A top right panel). However, symmetric F-actin rings could not be observed when LAD-III NK cells were stimulated with anti-CD16 (Figure 4A bottom right panel).
The CD16-mediated symmetric spreading is LFA-1– and kindlin-3–dependent. (A) Fluorescence images of F-actin in healthy donor NK cells or LAD-III NK cells stimulated on surfaces coated with anti-NKG2D (3.0 μg/mL) or anti-CD16 (5.0 μg/mL). (B) The percentage of healthy or LAD-III NK cells forming a peripheral ring of F-actin in response to surfaces coated with poly lysine and indicated concentrations of anti-CD16 (light blue and pink, respectively) or surfaces coated with ICAM-1 and indicated concentrations of anti-CD16 (blue and red, respectively). Graphs represent mean ± SEM; n = 60-90. (C) The area of cells attached to the slide surface, as assessed by IRM imaging, was measured for healthy and LAD-III NK cells on surfaces coated with ICAM-1 and increasing concentrations of anti-CD16 (blue and red, respectively) and for healthy and LAD-III NK cells on surfaces coated with poly lysine and increasing concentrations of anti-CD16 (light blue and pink, respectively). Graphs represent mean ± SEM; n = 60-90. (D) The distance from the centroid of healthy or LAD-III NK cells (cell activated using anti-CD16 and anti-NKG2D at 5.0 μg/mL) stained with phalloidin-ATTO-647N to the circumference was measured at 360 radii, and the symmetry of the cells was assessed by the SD of the radii (left) and the ratio of maximum and minimum lengths (right, n = 40-70). ***P < .0005 (ANOVA). **P < .005 (ANOVA). *P < .05 (ANOVA). NS indicates not significant.
The CD16-mediated symmetric spreading is LFA-1– and kindlin-3–dependent. (A) Fluorescence images of F-actin in healthy donor NK cells or LAD-III NK cells stimulated on surfaces coated with anti-NKG2D (3.0 μg/mL) or anti-CD16 (5.0 μg/mL). (B) The percentage of healthy or LAD-III NK cells forming a peripheral ring of F-actin in response to surfaces coated with poly lysine and indicated concentrations of anti-CD16 (light blue and pink, respectively) or surfaces coated with ICAM-1 and indicated concentrations of anti-CD16 (blue and red, respectively). Graphs represent mean ± SEM; n = 60-90. (C) The area of cells attached to the slide surface, as assessed by IRM imaging, was measured for healthy and LAD-III NK cells on surfaces coated with ICAM-1 and increasing concentrations of anti-CD16 (blue and red, respectively) and for healthy and LAD-III NK cells on surfaces coated with poly lysine and increasing concentrations of anti-CD16 (light blue and pink, respectively). Graphs represent mean ± SEM; n = 60-90. (D) The distance from the centroid of healthy or LAD-III NK cells (cell activated using anti-CD16 and anti-NKG2D at 5.0 μg/mL) stained with phalloidin-ATTO-647N to the circumference was measured at 360 radii, and the symmetry of the cells was assessed by the SD of the radii (left) and the ratio of maximum and minimum lengths (right, n = 40-70). ***P < .0005 (ANOVA). **P < .005 (ANOVA). *P < .05 (ANOVA). NS indicates not significant.
Quantification of these results across multiple experiments revealed that the ability of CD16 ligation to trigger formation of a peripheral F-actin ring was significantly impaired for LAD-III NK cells (Figure 4B). Intriguingly, LAD-III NK cell activation was rescued to a certain extent when slides were coated with both anti-CD16 and ICAM-1. The lack of spreading of LAD-III NK cells was also reflected in the fact that the surface area of the LAD-III NK cells remained small when only anti-CD16 was present (Figure 4C) and the cells did not readily spread symmetrically on anti-CD16 compared with healthy NK cells or NK cells stimulated on anti-NKG2D (Figure 4D). The median values of the max:min ratio increases from 1.6 in healthy cells to 2.3 in LAD-III cells when stimulated on surfaces coated with anti-CD16, whereas for anti-NKG2D the maximum/minimum ratio increases from 1.7 to 2.0 or the SD radii increases from 0.83 to 1.28 um for LAD-III NK cells stimulated on anti-CD16, whereas it does not significantly increase when LAD-III and healthy NK cells stimulated on anti-NKG2D were compared (0.88 and 1.0 μm, respectively). Thus, these results suggest that the threshold for CD16-mediated killing is less reliant on kindlin-3, as observed from comparing LAD-III NK cells with various healthy donors.
The adhesiveness under shear-force and migration of LAD-III NK cells are impaired
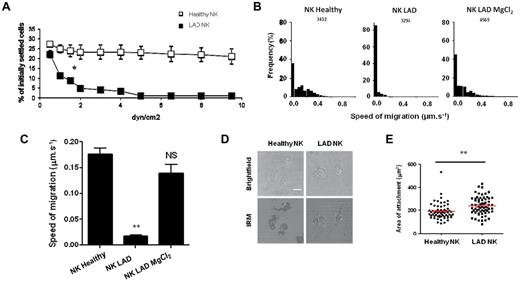
The ability of leukocytes to form adhesive interactions on endothelium under shear force is important for their extravasations into damaged tissue.37 Leukocyte integrins must undergo a conformational change to a high-affinity form to establish shear–resistant adhesion.37 It is known that kindlin-3 is required for the conformational switch13,14 and that our patient is defective in this switch.15 We therefore tested whether the LAD-III deficiency affects the NK cells ability to arrest on ICAM-1 under shear-stress conditions. As shown in Figure 5A, and in contrast to NK cells derived from various healthy controls (in Figure 5A 1 healthy control is presented), NK cells from the LAD-III patient were unable to adhere to ICAM-1 under shear-force conditions (supplemental Videos 1 and 2).
Spreading of LAD-III NK cells on ICAM-1 is intact, whereas shear-resistant adhesion and migration are impaired. (A) The figure shows the LFA-1–dependent adhesion under shear force. NK cells derived either from a healthy control (□) or from the LAD-III patient (■) were settled for 1 minute on ICAM-1-Fc–coated surfaces (350 sites/μm2) and were then subjected to increasing shear forces. Figure shows 1 representative experiment of 3 performed. Data are mean ± SD. Error bars (SD) are derived from triplicates. *P < .05. (B) Frequency histograms of the speed of cell migration on ICAM-1–coated slides measured at 5-second intervals, from healthy control NK cells or from the LAD-III patient NK cells, which had either been stimulated with 5mM MgCl2 or left untreated. The total number of time points analyzed for each experimental condition is indicated. (C) The average migration speeds of healthy control NK cells, LAD-III patient NK cells, or Mg2+ stimulated LAD-III patient NK cells on ICAM-1. Data are mean ± SEM, derived from 3 separate experiments. **P < .005. NS indicates not significant. (D) Bright-field and IRM images of NK cell surface attachment and spreading on ICAM-1 from a healthy control (left) or from the LAD-III patient (right). Scale bar represents 10 μm. (E) Quantification of the area of surface attachment for n > 60 cells. Data are mean ± SEM. **P < .005.
Spreading of LAD-III NK cells on ICAM-1 is intact, whereas shear-resistant adhesion and migration are impaired. (A) The figure shows the LFA-1–dependent adhesion under shear force. NK cells derived either from a healthy control (□) or from the LAD-III patient (■) were settled for 1 minute on ICAM-1-Fc–coated surfaces (350 sites/μm2) and were then subjected to increasing shear forces. Figure shows 1 representative experiment of 3 performed. Data are mean ± SD. Error bars (SD) are derived from triplicates. *P < .05. (B) Frequency histograms of the speed of cell migration on ICAM-1–coated slides measured at 5-second intervals, from healthy control NK cells or from the LAD-III patient NK cells, which had either been stimulated with 5mM MgCl2 or left untreated. The total number of time points analyzed for each experimental condition is indicated. (C) The average migration speeds of healthy control NK cells, LAD-III patient NK cells, or Mg2+ stimulated LAD-III patient NK cells on ICAM-1. Data are mean ± SEM, derived from 3 separate experiments. **P < .005. NS indicates not significant. (D) Bright-field and IRM images of NK cell surface attachment and spreading on ICAM-1 from a healthy control (left) or from the LAD-III patient (right). Scale bar represents 10 μm. (E) Quantification of the area of surface attachment for n > 60 cells. Data are mean ± SEM. **P < .005.
We next questioned whether LAD-III NK cells could migrate on ICAM-1–coated surfaces. In agreement with other publications studying other cell types13 and as opposed to migration of NK cells derived from a healthy control (similar results were obtained with additional healthy controls), we observed that LAD-III NK cells were unable to migrate on ICAM-1 (Figure 5B-C; supplemental Videos 3 and 4). Consistent with the observations that kindlin-3 is required for optimal conformational activation of integrins,14,15,19 on treatment of the cells with Mg2+ (which causes an artificial integrin activation through a conformational switch to their high-affinity form38 ), the LAD-III NK cells were now capable of migrating on ICAM-1 (Figure 5B-C; supplemental Video 5). The observation that LAD-III NK cells could not migrate was in agreement with other cell types studied.13,14 However, in contrast to other cell types, we had observed by fixed cell imaging that LAD-III NK cells had a normal migratory morphology on ICAM-1 (Figure 3A; supplemental Figure 7). To confirm this, we quantified the surface area of live NK cells in contact with ICAM-1 coated slides, assessed by IRM imaging. We found that, as for fixed cell imaging, live LAD-III NK cells on ICAM-1 had a similar migratory morphology to healthy NK cells (Figure 5D) and formed a contact area with the slide surface, which was indeed slightly larger than that for healthy cells (Figure 5E). This is probably because the leading edge is mobile and extends out, even though the LAD-III NK cells are unable to move. Similar results were obtained with NK cells derived from various healthy controls. This suggests that formation of a mobile leading edge and polarized morphology in NK cells is kindlin-3–independent whereas optimal migration is kindlin-3–dependent.
Kindlin-3 is dispensable when multiple NK cell receptors are cotriggered
We observed above that the killing of Daudi cells by the LAD-III–deficient NK cells is similar to that of healthy controls (Figure 2D) but that the killing of the kindlin-3–deficient NK cells is impaired when single receptor is triggered (Figure 2A-C). We therefore next tested the ability of LAD-III NK cells to kill target cells expressing multiple activating ligands (supplemental Figure 8). We initially tested the killing of the ICAM-1–positive cell line, 1106mel (supplemental Figure 9) and observed that, similar to the killing of the Daudi cells (Figure 2D), the cytotoxicity of LAD-III NK cells against 1106mel cells was intact and comparable to the killing of a healthy control (Figure 6A). Similar results were obtained with other healthy controls (summarized in supplemental Figure 10).
NK cell cytotoxicity is LFA-1–dependent, kindlin-3–independent. (A) Bulk NK cells derived from a healthy control or from the LAD-III patient were assayed for killing of the 1106mel melanoma cell line. The various NK cells were either left untreated or treated with anti-LFA-1 mAb (168.6), XVA, (a β2-integrin chemical blocker), or with a control mAb (12E7). The E:T ratio was 5:1. Figure shows 1 representative experiment of 6 performed. Data are mean ± SD. Error bars (SD) are derived from triplicates. *P < .05. NS indicates not significant. (B) NK cells derived from the LAD-III patient and from a healthy control were incubated for 2.5 hours with 1106mel cells at 37°C with and without the various reagents described in panel A. The expression of CD107a was analyzed by FACS on the CD56+ cells. The E:T ratio was 1:1. Data are mean ± SD. Error bars (SD) are derived from triplicates. *P < .05. NS indicates not significant. Figure shows 1 representative experiment of 2 performed. (C) Bulk NK cells derived from a healthy control or from the LAD-III patient were assayed for killing of various target cells (indicated above the graphs) at different E:T ratios (as indicated on the x-axis). The NK cells were treated with anti-LFA-1 mAb (168.6) or with a control mAb (12E7). Figure shows 1 representative experiment of 2 performed. Data are mean ± SD. Error bars (SD) are derived from triplicates. *P < .05. **P < .005. ***P < .0005.
NK cell cytotoxicity is LFA-1–dependent, kindlin-3–independent. (A) Bulk NK cells derived from a healthy control or from the LAD-III patient were assayed for killing of the 1106mel melanoma cell line. The various NK cells were either left untreated or treated with anti-LFA-1 mAb (168.6), XVA, (a β2-integrin chemical blocker), or with a control mAb (12E7). The E:T ratio was 5:1. Figure shows 1 representative experiment of 6 performed. Data are mean ± SD. Error bars (SD) are derived from triplicates. *P < .05. NS indicates not significant. (B) NK cells derived from the LAD-III patient and from a healthy control were incubated for 2.5 hours with 1106mel cells at 37°C with and without the various reagents described in panel A. The expression of CD107a was analyzed by FACS on the CD56+ cells. The E:T ratio was 1:1. Data are mean ± SD. Error bars (SD) are derived from triplicates. *P < .05. NS indicates not significant. Figure shows 1 representative experiment of 2 performed. (C) Bulk NK cells derived from a healthy control or from the LAD-III patient were assayed for killing of various target cells (indicated above the graphs) at different E:T ratios (as indicated on the x-axis). The NK cells were treated with anti-LFA-1 mAb (168.6) or with a control mAb (12E7). Figure shows 1 representative experiment of 2 performed. Data are mean ± SD. Error bars (SD) are derived from triplicates. *P < .05. **P < .005. ***P < .0005.
LAD-III NK cell cytotoxicity was LFA-1–dependent because the addition of a specific mAb directed against LFA-1, or the inclusion of XVA143 (a small allosteric blocker of β2 integrin) blocked lysis (Figure 6A). A similar picture was observed when assaying degranulation of LAD-III NK cells compared with a healthy control (Figure 6B). Furthermore, IFN-γ production of LAD-III NK cells in response to incubation with 1106mel cells was also similar to that of a healthy control (supplemental Figure 11) but interestingly not LFA-1–dependent (supplemental Figure 11).
Because LAD-III NK cells killed 1106mel (Figure 6) and Daudi (Figure 2) to a similar extent as healthy NK cells and because this killing was LFA-1–dependent, we were intrigued to test whether the killing of other targets will also not be affected by the absence of kindlin-3. Several cell lines expressing ICAM-1 (supplemental Figure 9B) were tested for killing by LAD-III and healthy NK cells. All target cell lines tested were similarly killed by LAD-III and by healthy NK cells and the killing was LFA-1–dependent (Figure 6C). Similar results were obtained with additional healthy donors. Thus, when multiple NK cell receptors are engaged, NK cell killing of target cells is LFA-1–dependent and kindlin-3–independent.
Discussion
Only recently, it has been proposed that mutations in kindlin-3 are the cause for LAD-III deficiency.12-18 Experiments performed on cells derived from LAD-III–deficient patients determined that kindlin-3 is essential for integrin activation in different cells.9,13-16,39 Kindlin-3 deficiency resulted in impairment of many integrin activities, including adhesion, spreading, aggregation, and migration.13,14 Because integrins, and especially LFA-1, play a central role in NK cell activation,40 we set out to test whether the absence of kindlin-3 will affect NK cell activities.
For this purpose, we obtained NK cells from a recently identified LAD-III patient15 and examined various NK cell functions. Because of ethical reasons, we could not obtain sufficient amounts of fresh NK cells from the patient; therefore, it is important to note that the NK cells used in all functional experiments were IL-2 activated. This level of activation could make the phenotype appear milder than it might be in vivo.
The patient NK cells were examined during a period of 2 years (1-3 years of age), and the activity of the NK cells was compared with many different healthy controls of different ages and genders during this time period. Because of ethical reasons, we were not able to obtain blood from healthy 1- to 3-year-old infants. However, from our experience in the clinic and from published data,41 we know that NK cell cytotoxicity is similar between adults and 1- to 3-year-old children.
We found that LAD-III NK cells do not migrate on ICAM-1–coated surfaces as demonstrated in all other cell types and in all LAD-III patients described so far.13-16 Importantly, and in agreement with other reports demonstrating that the conformational change of integrin is impaired in the LAD-III–deficient patient,14,15 on treating the LAD-III NK cells with Mg2+, they regained their migratory capability.
In NK cells, the nature and number of ligands for NK cell receptors determine target cell sensitivity to killing.42 It was shown that individual NK cell receptors, such as NKG2D or 2B4, induce inside-out signals that lead to activation of LFA-1,36 but coengagement of LFA-1, 2B4, and NKG2D defines the minimal requirement for NK cell natural cytotoxicity of resting NK cells.36 Here we demonstrate that kindlin-3 is especially important when NK cells are activated through a single receptor (ie, in the case of minimal triggering).
We have previously shown that stimulation of NK cells by ICAM-1 results in cells adopting an asymmetric morphology, as a migrating cell.31 As we have shown here, LAD-III NK cells are capable of forming a migratory morphology but are not able to migrate on ICAM-1. For activation and efficient cytotoxicity, an NK cell must initially stop migrating, symmetrically spread, and polymerize F-actin. When we stimulated the LAD-III NK cells with both ICAM-1 and MICA, we observed that LAD-III NK cells require a higher threshold of MICA to acquire a symmetric activated morphology. Multiple signals regulate NK cells,43 and whether NK cells will be activated or inhibited is dependent on the integration of all of these signals.43 We therefore suggest that kindlin-3 is important in establishing a threshold for the “stop” signal delivered by MICA and in the absence of kindlin-3 this threshold is elevated. This is consistent with NKG2D-mediated killing being less efficient but not completely impaired in LAD-III NK cells.
Interestingly, we observed that LAD-III NK cells produced symmetric cell morphology when stimulated though NKG2D but not when stimulated through CD16. This may be explained by the strong reliance on LFA-1 activation in ADCC demonstrated by previous studies which show that efficient ADCC (which is CD16–mediated) by resting NK cells requires the combined presence of IgG and expression of human ICAM-1,44 and that CD16 induces the highest levels of LFA-1 activation through inside-out signaling.36
Our data indicate that the function of CD16 is quite substantially dependent on kindlin-3 because LAD-III NK cells were unable to form a symmetric morphology, even with high concentrations of anti-CD16. Interestingly, when stimulating NK cell with both anti-CD16 and ICAM-1, we observed that LAD-III NK cells presented similar levels of NK cells with an F-actin ring as NK cells from healthy controls. Thus, simultaneous stimulation of CD16 and LFA-1 repairs the ability of LAD-III NK cells to be activated. This is consistent with the overall interpretation of these data that kindlin-3 influences the threshold at which NK cells are activated but does not entirely abrogate their ability to respond.
LAD-III NK cells killed various target cells at a similar manner as healthy NK cells; and although the killing was LFA-1–dependent, it did not require kindlin-3. Interestingly, when stimulating NK cell killing through one NK cell receptor, we observed impairment in NK cell cytotoxicity. The major difference between these assays is that the various target cells used express various ligands for different NK cell receptors, whereas, in the redirected killing assays, in ADCC and in the JEG3-transfected cells only one receptors is engaged. Taking these results together, we conclude that when multiple NK cell receptors are involved, the multiple signals are capable of overcoming the kindlin-3 deficiency, probably because they constitute a higher threshold for NK cell activation or can substitute for the weaker integrin adhesiveness of kindlin-3–deficient cells. Nevertheless, LFA-1 is still essential for NK cell killing of target cells, even when multiple NK receptors are co-triggered possibly because of its indispensible role in the proper polarization of the cytolytic NK machineries in the synapse of the NK and target cell.
NK cells express inhibitory receptors, and it is proposed that they are better equipped to stop granule polarization than to block degranulation.45 It will be interesting to test in the future whether the absence of kindlin-3 alters the threshold for NK cell inhibition.
Interestingly, only recently it was demonstrated in neutrophils that talin-1 and kindlin-3 are involved in distinct steps of conformational integrin activation.46 It was demonstrated that both talin-1 and kindlin-3 are needed to induce the high-affinity LFA-1 conformation and neutrophil arrest. However, the mechanisms by which talins and kindlins are recruited and bind β-integrin cytoplasmic tails remain poorly understood, and it would be interesting to further investigate them in the future. Further, it is well established that Vav1 is required for accumulation of actin at the NK-target site in response to NKG2D signaling.47 Thus, it would also be interesting to investigate the role of kindlin-3 in the immune synapse and the relationship between kindlin-3 and Vav1.
Whether the clinical symptoms observed in this LAD-III patient are associated with her NK cell dysfunctions is hard to tell. Our patient (like other LAD-III patients) has recurrent bacterial infection, and NK cells are known to play an essential role in controlling bacterial infections.48 However, at this point, we cannot directly link the NK cell deficiencies with the increased bacterial infections observed.
One of the major abnormalities seen in the LAD-III patients is the increased numbers of lymphocytes in the blood (∼ 10 times than normal). The novel observations seen here might be of medical importance; once a treatment will be found that will restore the NK cell adhesion under shear force, we predict that these LAD-III NK cells will be able to kill invading pathogens when multiple NK cell killing receptors are engaged.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Israeli Science Foundation, the Israel Cancer Research Foundation (professorship grant), the MOST-DKFZ (research grant), the Rosetrees Trust, the I-CORE Program of the Planning and Budgeting Committee, the Katten Foundation, and the Association for International Cancer Research (all to O.M); and the Medical Research Council (United Kingdom), the Biotechnology and Biologic Sciences Research Council, and a Royal Society Wolfson Research Merit Award (D.M.D.). O.M. is a Crown Professor of Molecular Immunology.
Authorship
Contribution: R.G. performed NK phenotyping experiments and cytotoxicity experiments, analyzed the data, and wrote the manuscript; A.C.N.B. performed all NK spreading, migration, and activation experiments and analyzed the data; V.G. and S.W.F. performed the shear-force experiments; S.M. produced the anti-LFA-1 hybridoma; C.G. provided critical reagents; H.A. and Y.B. produced the JEG3 transfectants; R.A. supervised the work; M.A. recruited the patients and medically treated the patients; and D.M.D. and O.M. supervised the project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for H.A. is Department of Infectious Diseases, Israel Institute for Biological Research, Ness-Ziona, Israel.
V.G. died on October 10, 2010.
Correspondence: Ofer Mandelboim, Lautenberg Center for General and Tumor Immunology, Hebrew University Hadassah Medical School, Institute for Medical Research Israel-Canada, Hadassah Ein Karem, Jerusalem, Israel 91120; e-mail: oferm@ekmd.huji.ac.il.
References
Author notes
R.G. and A.C.N.B. contributed equally to this study.
D.M.D. and O.M. contributed equally to this study.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal