Abstract
HOX proteins are widely involved in hematopoietic development. These transcription factors combine a conserved DNA-binding homeobox with a divergent N-terminus that mediates interaction with variable cofactors. The resulting combinatorial diversity is thought to be responsible for mammalian HOX specificity. Contrasting this proposed mechanism for normal HOX function, here we demonstrate that, in the context of hematopoietic immortalization and leukemogenesis, individual HOX properties are governed almost exclusively by the homeodomain. Swap experiments between HOXA1 and HOXA9, 2 members of nonrelated paralog groups, revealed that gene expression patterns of HOX transformed cells in vitro are determined by the nature of the homeodomain. Similar results were seen in vivo during HOX-mediated leukemogenesis. An exchange of the homeodomains was sufficient to convert the slow, low-penetrance phenotype of HOXA1-induced leukemia to the aggressive fast-acting disease elicited by HOXA9 and vice versa. Mutation and deletion studies identified several subregions within the DNA binding domain responsible for paralog specificity. Previously defined binding sites for PBX cofactors within the exchangeable, nonhomeobox segment were dispensable for in vitro oncogenic HOX activity but affected in vivo disease development. The transcriptional activator domain shared by HOXA1 and HOXA9 at the very N-terminus proved essential for all transformation.
Introduction
Next to their role in embryo patterning, HOX genes also play an eminent role during normal and malignant hematopoiesis.1-3 The mammalian HOX family is composed of 39 members that are clustered in 4 groups (A-D). Each contains between 9 and 11 different HOX paralogs classified by the homeodomain. Hematopoietic stem cells and early precursors mainly express HOX genes of groups A and B. During differentiation, these genes are gradually switched off in order of their genomic location. The “anterior” HOX genes corresponding to the antennapedia complex in Drosophila (HOX1-8 at the 3′ end of the cluster with respect to the transcriptional orientation) are extinguished before the “posterior” genes (HOX9-13 at the 5′ end of the cluster). Mature hematopoietic cells generally do not express HOX genes any more.
Increased HOX activity blocks hematopoietic differentiation and immortalizes highly proliferative progenitors. This preleukemic population can be converted to overt leukemia by additional mutations. Therefore, it is not surprising that HOX genes and in particular the “abdominal”-type members are involved in leukemogenesis at multiple instances. A general overexpression of all HOX genes is observed in many cases of acute myeloid leukemia. In clinical samples, HOXA9 has been shown to be a negative prognostic factor with survival inversely correlated to HOXA9 expression.4 The HOX-A cluster is also involved in acute T-cell leukemia where a chromosomal translocation brings part of this genomic locus under control of the strong enhancer normally driving the T-cell receptor.5,6 Furthermore, HOX genes participate in translocations that create highly leukemogenic HOX fusions with proteins of the nuclear pore complex.7 In addition, induction of HOX expression is the central mechanism of blood cell transformation caused by MLL fusion proteins. MLL, a trithorax protein, is an upstream regulator of HOX activity controlling HOX loci by chromatin modification. Oncogenic MLL fusions thwart this normal function and cause constitutive HOX expression.8-10 Similarly, other HOX upstream regulators, such as the CDX proteins, are found at higher than normal levels in acute leukemia.11-14 And finally, 2 very recent reports point out an important role for HOXA9 during blast crisis in chronic myeloid leukemia15 and in a subgroup of multiple myeloma.16
At the structural level, HOX proteins are transcription factors that display a bipartite composition consisting of a highly divergent N-terminus, including sequences with transcriptional activator function17 followed by a C-terminal DNA binding motif termed homeobox. This highly conserved domain is the common denominator of all HOX proteins and defines the phylogenetic relationship of different HOX paralog groups. Short AT-rich sequences were determined in site selection and other in vitro experiments as the preferred binding site for HOX proteins.18-21 As these motifs are very numerous in DNA, additional specificity is required. Therefore, significant advance was made when it was realized that, in analogy to Drosophila, also mammalian HOX proteins form cooperative DNA binding complexes with other homeodomain proteins of the PBX (extradenticle in fly) and MEIS (homothorax) families.22-27 Mammals possess 5 homothorax related genes (MEIS1-3, PKNOX1, PKNOX2) and 4 PBX genes that are each transcribed in various isoforms. Therefore, HOX/MEIS/PBX complexes can occur in a great combinatorial diversity. The importance of MEIS as HOX cofactor was corroborated by experiments in leukemia prone BXH2 mice where malignant cells frequently carried retroviral insertions coactivating Meis1 (hence, the name myeloid ecotropic viral integration site) and either Hoxa9 or Hoxa7.28 In addition, it was shown several times that efficient leukemogenesis in experimental animals requires overexpression of a Hox gene together with Meis.29-31 The question of whether PBX proteins are necessary for HOX-mediated leukemogenesis is not completely settled yet. It was reported that coexpression of Pbx1b does not cooperate with Hoxa9 in hematopoietic transformation29 and that deletion of a conserved Pbx interaction motif in either Hoxa9 or a NUP98-HOXA9 fusion leaves transforming activity untouched.32,33 In contrast, other publications find the same motif essential for oncogenic activity17 and describe a reduction of transformation potential for MLL fusion proteins (that work through up-regulation of Hox genes) in cells with reduced Pbx2 and Pbx3.31
Recently, we characterized the leukemogenic potential of each individual HOXA gene.34 In this study, we could demonstrate that, apart from the known oncogenes of the “posterior,” abdominal B-type genes also “anterior” labial-like HOX proteins have significant transforming capacity and cooperate with Meis1. However, the phenotype of the occurring leukemia was different. HOXA1 elicited a long-latency disease with reduced penetrance, and leukemic cells displayed myeloid differentiation markers. In contrast, HOXA9 caused a rapidly lethal, more stem-cell-like leukemia with 100% penetrance. To elucidate the basis for these observed differences, here we performed a structural study delineating the “specificity determinants” of HOXA1 and HOXA9. Unexpectedly, we found that the homeodomain alone is the major determinant of paralog specific features in all aspects tested. In contrast, the completely nonsynonymous N-termini of HOXA1 and HOXA9 were functionally equal. Targeting this common denominator might provide a possibility for a coordinated “anti-HOX” therapy instead of targeting HOX members individually.
Methods
Plasmids, retroviral constructs, cell culture, antibodies
All HOX constructs and derivatives thereof were cloned by PCR using either human HOXA1 (NM_005522.4) or HOXA9 (NM_152739.3) as template. Amplicons were inserted into the pMSCV retroviral vector series35 (Clontech, TaKaRa) and supplemented with an N-terminal HA-tag to aid immunologic detection. For luciferase assays, GAL4 DNA-binding domain fusions were generated in the vector pSG424.36 All clones were sequenced to avoid inadvertent introduction of errors by Taq polymerase. A murine pMSCVneo-Meis1 plasmid was a laboratory stock. Retroviral packaging was done in the Phoenix-E packaging line.37 Antibodies for Western detection and FACS were from Sigma-Aldrich or BD Biosciences.
Replating assays
Replating or colony formation cell assays were done essentially as described.38,39 This assay allows assessment of the clonogenic capacity of hematopoietic precursor cells after repeated replating in semisolid medium. An enhanced self-renewal capacity is detected as surrogate parameter for transformation activity. In short, hematopoietic precursor cells were isolated from the bone marrow of 8- to 12-week-old Balb/c mice by magnetic selection for CD117 (c-kit) according to the instructions of the manufacturer (Miltenyi Biotec). The cells were activated in the presence of recombinant murine IL-3, IL-6 (10 ng/mL), and SCF (100 ng/mL). After 2 rounds of retroviral infection by spinoculation (2.5 hours at 34°C and 2500g), cells were seeded in methocel media (M3234, StemCell Technologies) under appropriate selective conditions and with cytokines as in activation medium plus an addition of GM-CSF at 10 ng/mL. Colony counts were recorded after 2 rounds of replating. For the generation of permanent HOX-transformed lines, cells from the second replating were transferred to RPMI 1640 medium supplemented with the same cytokines.
Transplantation experiments
For induction of acute leukemia in syngenic recipients, 1 × 106 transduced cells were transplanted together with 1 × 106 normal bone marrow cells as radioprotectant into lethally irradiated (9 Gy) animals by intravenous injection. Animals were monitored on a daily basis. After first signs of overt disease were visible, the recipients were killed and a postmortem analysis was performed. Permanent cell lines were regrown in cytokine supplemented media from leukemic spleen. Engraftment of HOXA9ΔPBX/Meis cells was confirmed by PCR on genomic DNA isolated from peripheral blood of transplanted animals with primers ΔPBXfw:atgcttgtggttctcctccagttg, and ΔPBXrev: ccagggtctggtgttttgtatagg. To avoid coamplification of the highly homologous mouse Hoxa9 sequence, these primers were chosen to span intron 1 of endogenous Hoxa9. All animal procedures were performed according to the regulations of the local and institutional authorities (Federation for Laboratory Animal Science Associations [FELASA] recommendations, University of Erlangen Department of Animal welfare, regional licensing authority, license #TS-99/01, 621.2531.32-03/00, 54-2532.2-5/12).
Quantitative RT-PCR, microarray experiments, luciferase assays
Quantitative RT-PCR was performed by SYBR Green–based chemistry (Stratagene) on a MX3000P real-time cycler according to the instructions of the manufacturer. Primers were designed such that the resulting amplicons spanned introns. Primers used were: β-actin(forward): ccaactgggacgacatggag; β-actin(reverse): ctcgtagatgggcacagtgtg; Myb (forward): gaataaaggagctggagttgctc, Myb (reverse): gtgcatctaagcccgagctttc; Slc14a1(forward): cagccacaggacactacaatac, Slc14a1 (reverse): gttgaaaccccagagcccaaag; Enpp1 (forward): ctggttttgtcagtatgtgtgct, Enpp1(reverse): ctcaccgcacctgaatttgttg. Relative expression values were determined by the 2−ΔΔCt method using β-actin as normalizer.
Microarray experiments were performed commercially by AtlasBiolabs GmbH. RNA from 2 biologic replicates per HOX construct was pooled and hybridized to Agilent 44K mouse expression arrays. Full raw data have been deposited at ArrayExpress under accession number E-MEXP-3617. Principal component analysis was performed using the 10 most differentially expressed genes that discriminate HOXA1- and HOXA9-transformed cells: for HOXA1, Scin, Gzmb, Col18a1, Crhbp, and Ptprz1; and for HOXA9, Gpx8, Ly6f, Airn, Cyp26a1, and Ccl8. Expression normalized to actin was determined for each gene in 3 independent RNA samples. These values were scaled to relative expression across all samples, and principal components were built by addition of relative expression units.
Luciferase assays were done by electroporation of REH cells according to standard protocols. A construct containing a minimal SV40 promoter preceded by a triplicate of the GAL4 binding site served as reporter (derived from pGL3-basic, Promega). Electroporations were done in triplicates with 0.1 μg reporter plasmid and 0.9 μg pSG424-based expression construct.
Where appropriate, a Student t test was performed to probe for statistical significance which was assumed for P values < .05.
Immunoprecipitation
For immunoprecipitation of HOX, HOX derivatives, and PBX, epitope-tagged versions were coexpressed in 293T cells. Total cell extracts were prepared in lysis buffer (20mM HEPES, pH 7.5, 10mM KCl, 0.5mM EDTA, 0.1% Triton X-100, 10% glycerol, 300mM NaCl, supplemented with 1mM PMSF). Because efficient heterodimerization of HOX and PBX proteins requires DNA, 0.1 nmol of a double-stranded oligo corresponding to a HOX/PBX consensus (ctgcgatgatttacgaccgc) was added to total lysates. Precipitation was done with antiflag agarose; and after thorough washing in lysis buffer, the precipitated material was analyzed by immunoblot.
Results
Design and functionality of swap constructs
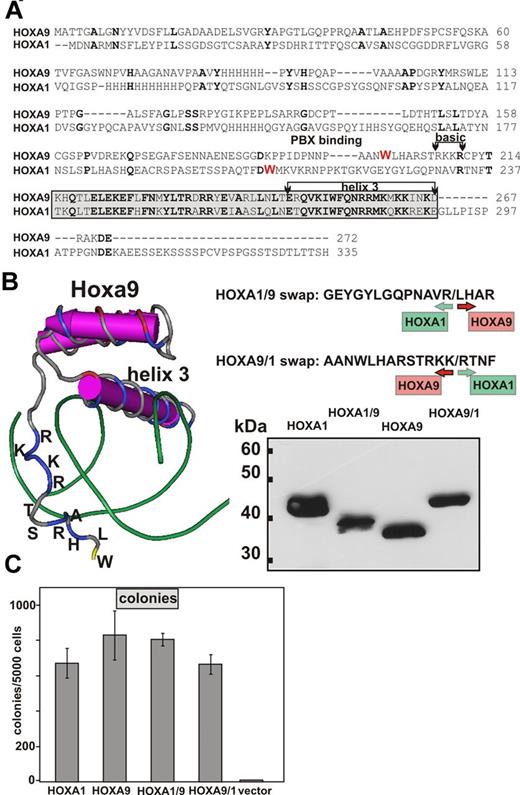
To delineate the respective contribution of the 2 major HOX components toward HOX specific function, we constructed swap clones exchanging the homeodomain of HOXA1 with that of HOXA9 and vice versa (Figure 1). The homeobox of HOXA9 is preceded by an N-terminal extension that contains 4 basic amino acids (RKKR; Figure 1A). A crystal structure of HOXA9 has been solved.40 From these data, it can be inferred that this basic tetrad is positioned close to the minor groove opposite of the actual DNA recognition helix 3 (Figure 1B). Because this motif may increase general protein-DNA affinity, it was grafted together with some flanking amino acids and the actual core-homeodomain onto the N-terminus of HOXA1 to create a HOXA1/9 swap. This particular design leaves the vestigial arginine of the “basic tetrad” in HOXA1 intact and creates a small “duplication.” However, this feature of HOXA1 is moved upwards; and as suggested by the structure of the HOXA9 homeodomain, it is likely out of reach to make further contacts with DNA. No structural data are available for HOXA1. Therefore, a linear design was chosen for the reciprocal HOXA9/A1 swap, replacing all amino acids downstream of the conserved R210 in HOXA9 (R233 in HOXA1) with the respective residues of HOXA1. The chimeric HOX genes were cloned into a pMSCV retroviral backbone and tested in phoenix packaging cells for expression. Similar to the parental HOXA1 and HOXA9 constructs, all swaps carried an N-terminal HA-tag to aid detection by Western blot. All constructs were expressed, correctly indicating that swapping the homeodomain does not impair protein stability. To test the functionality of the modified HOX proteins, hematopoietic precursor cells were transduced with the respective swaps and parental constructs as controls (Figure 1C). Transduced cells were analyzed in replating assays. All constructs showed in vitro transforming activity enhancing self-renewal capability of primary hematopoietic cells. We consistently observed a small reduction in colony numbers for cells transduced by HOXA1 compared with HOXA9, probably reflecting the weaker transforming ability of HOXA1.34 Interestingly, this effect was correlated with the homeodomain. Grafting the HOXA9 homeodomain onto HOXA1 augmented the transforming capability of the HOXA1/9 chimera, whereas the reciprocal fusion blunted the response in the replating assay to a level reached with the parental HOXA1 construct. This phenomenon prompted us to investigate the underlying molecular principles.
Design, expression, and functionality of HOX swap constructs. (A) BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi) alignment of human HOXA1 and HOXA9 proteins. Amino acid sequences are given in the 1-letter code. Identical residues are in bold type. The tryptophan essential for interaction with PBX cofactors is marked in red. The amino-terminal basic extension and the core homeodomain, including recognition helix 3, are highlighted. (B) Left panel: 3-dimensional structure of a murine Hoxa9/Pbx1 dimer bound to DNA (protein structure database accession: 1PUF). Only part of Hoxa9 and the DNA helix are shown. For the N-terminal extension upstream of the core homeodomain, the respective amino acids are given in the 1-letter code. Green represents DNA, and amino acids are colored according to their charge: blue represents basic; yellow, hydrophobic; and red, acidic. This figure is a screenshot of a visualization done with the software Cn3D (http://www.ncbi.nlm.nih.gov/Structure/CN3D/cn3d.shtml). Top right panel: Junction sequences of the swap clones with their respective HOXA1 and HOXA9 contributions. Bottom right panel: Immunoblot of chimeric HOX proteins and their parental counterparts. Nuclear extracts were analyzed from Phoenix-E packaging cells transfected with the respective retroviral plasmids. After separation by SDS-PAGE, proteins were blotted and detected by an anti-HA tag antibody. (C) Colony counts obtained in replating assays. Primary, hematopoietic precursors were transduced with the constructs as indicated and replated twice in cytokine containing methocel media. Colony numbers are given as mean ± SD of 3 biologic replicates.
Design, expression, and functionality of HOX swap constructs. (A) BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi) alignment of human HOXA1 and HOXA9 proteins. Amino acid sequences are given in the 1-letter code. Identical residues are in bold type. The tryptophan essential for interaction with PBX cofactors is marked in red. The amino-terminal basic extension and the core homeodomain, including recognition helix 3, are highlighted. (B) Left panel: 3-dimensional structure of a murine Hoxa9/Pbx1 dimer bound to DNA (protein structure database accession: 1PUF). Only part of Hoxa9 and the DNA helix are shown. For the N-terminal extension upstream of the core homeodomain, the respective amino acids are given in the 1-letter code. Green represents DNA, and amino acids are colored according to their charge: blue represents basic; yellow, hydrophobic; and red, acidic. This figure is a screenshot of a visualization done with the software Cn3D (http://www.ncbi.nlm.nih.gov/Structure/CN3D/cn3d.shtml). Top right panel: Junction sequences of the swap clones with their respective HOXA1 and HOXA9 contributions. Bottom right panel: Immunoblot of chimeric HOX proteins and their parental counterparts. Nuclear extracts were analyzed from Phoenix-E packaging cells transfected with the respective retroviral plasmids. After separation by SDS-PAGE, proteins were blotted and detected by an anti-HA tag antibody. (C) Colony counts obtained in replating assays. Primary, hematopoietic precursors were transduced with the constructs as indicated and replated twice in cytokine containing methocel media. Colony numbers are given as mean ± SD of 3 biologic replicates.
HOX-specific gene expression patterns are determined by the homeodomain
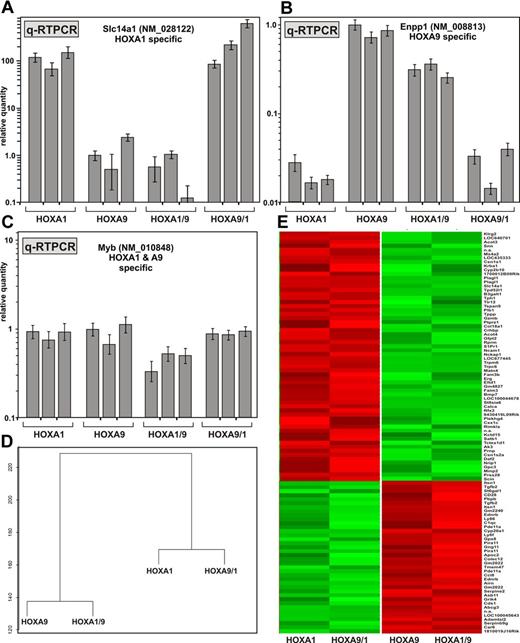
Because differences in phenotype should have their reflection in a particular genotype, we analyzed paralog-specific gene expression (Figure 2). First, we concentrated on sentinel genes that are exclusively induced either by HOXA1, HOXA9, or by both proteins as previously identified in our laboratory (C.B. and R.K.S., unpublished data, 2011). The gene for the solute carrier protein Slc14a1 (NM_028122) is specifically expressed in HOXA1-transformed cells at levels 100- to 1000-fold higher than in HOXA9 cells. Enpp1 (NM_008813) encoding ectonucleotide pyrophosphatase/phosphodiesterase 1 shows the opposite behavior with expression levels ∼ 30-fold higher in HOXA9-transformed cells. Myb (NM_010848) is a direct downstream target of both HOXA1 and HOXA9 and is expressed approximately at equal levels in cells transformed by these genes.34,41 Primary hematopoietic precursor cells were transduced with HOXA1, HOXA9, HOXA1/9, or HOXA9/1 in 3 independent experiments. After 2 rounds of replating, cells were transferred to liquid culture and expanded into cell lines. RNA was isolated from 3 independent lines generated for each construct and analyzed by quantitative RT-PCR (Figure 2A-C). Remarkably, gene expression was only dependent on the nature of the homeodomain. Changing the HOXA1 homeodomain to that of HOXA9 altered sentinel gene expression to a pattern characteristic to the parental HOXA9 prototype and vice versa. As expected, Myb levels were not significantly affected by the nature of the homeodomain.
HOX-specific gene expression patterns are determined by the homeodomain. (A) Three independent cell lines were generated from primary bone marrow cells transformed by HOXA1, HOXA9, or the respective swap-constructs as indicated. RNA was harvested and quantitative RT PCR for Slc14a1 (NM_028122), a sentinel gene for HOXA1-transformed cells, was performed. Values were normalized for β-actin. Relative quantities are plotted as mean ± SD of a triplicate experiment. Expression levels found in the first HOXA9 transformed line were arbitrarily set to 1 unit. (B) Quantitative RT-PCR for Enpp1 (NM_008813), a gene specifically expressed in cells immortalized by HOXA9. Procedure and legend as in panel A. (C) Quantitative RT-PCR for Myb that is induced by HOXA1 and HOXA9. (D) Relationship of the gene expression program induced by parental HOX genes and the respective swap derivatives. RNA from the individual cell lines as described in panel A was pooled and hybridized to expression arrays. HOXA1 and HOXA9/1 patterns were compared with those found in HOXA9 and HOXA1/9 cells. The top 100 scoring genes with the highest expression difference and lowest variation (P < .05) were used to construct a dendrogram. (E) Clustering of the top scoring genes as in panel D. Genes are arranged as lines and RNA samples are represented by columns. The transduced HOX construct is given at the bottom. A listing of the individual expression values can be found in supplemental Table 1.
HOX-specific gene expression patterns are determined by the homeodomain. (A) Three independent cell lines were generated from primary bone marrow cells transformed by HOXA1, HOXA9, or the respective swap-constructs as indicated. RNA was harvested and quantitative RT PCR for Slc14a1 (NM_028122), a sentinel gene for HOXA1-transformed cells, was performed. Values were normalized for β-actin. Relative quantities are plotted as mean ± SD of a triplicate experiment. Expression levels found in the first HOXA9 transformed line were arbitrarily set to 1 unit. (B) Quantitative RT-PCR for Enpp1 (NM_008813), a gene specifically expressed in cells immortalized by HOXA9. Procedure and legend as in panel A. (C) Quantitative RT-PCR for Myb that is induced by HOXA1 and HOXA9. (D) Relationship of the gene expression program induced by parental HOX genes and the respective swap derivatives. RNA from the individual cell lines as described in panel A was pooled and hybridized to expression arrays. HOXA1 and HOXA9/1 patterns were compared with those found in HOXA9 and HOXA1/9 cells. The top 100 scoring genes with the highest expression difference and lowest variation (P < .05) were used to construct a dendrogram. (E) Clustering of the top scoring genes as in panel D. Genes are arranged as lines and RNA samples are represented by columns. The transduced HOX construct is given at the bottom. A listing of the individual expression values can be found in supplemental Table 1.
To get a more comprehensive picture of the gene expression associated with a particular homeodomain, global expression profiling was done (Figure 2D-E). RNA was pooled from 2 biologic replicates according to the HOX gene used for transduction and hybridized to expression arrays. For evaluation, the HOXA1 and HOXA9/1 samples were compared with HOXA9 and HOXA1/9 cells. Approximately 4600 genes showed a ≥ 2-fold difference in expression levels between HOXA1 and HOXA9/1 versus HOXA9 and HOXA1/9. For the top scoring 100 genes with the largest expression changes and lowest variation (P < .05) a dendrogram and a heat map were developed (Figure 2D-E; supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Corroborating sentinel gene experiments, expression patterns in transformed cells clearly clustered with the respective homeodomain. To expand this analysis, we determined Pearson correlation coefficients for a larger dataset composing all 254 genes with a > 10-fold average difference in expression between HOXA1 and HOXA9 and a significant expression of > 2 log2 transformed hybridization value (supplemental Figure 1; supplemental Table 2). As expected, a comparison of HOXA1 and HOXA9 scored low (r = −0.05). In contrast, the RNA profile in the HOX9/1 swap cell lines was highly similar to that of HOXA1 cells (r = 0.81). Correlation between cells transformed by HOXA1/9 and the original HOXA9 constructs even reached a score of r = 0.90, indicating a nearly perfect match of the respective gene expression patterns.
The homeodomain is responsible for the leukemic phenotype
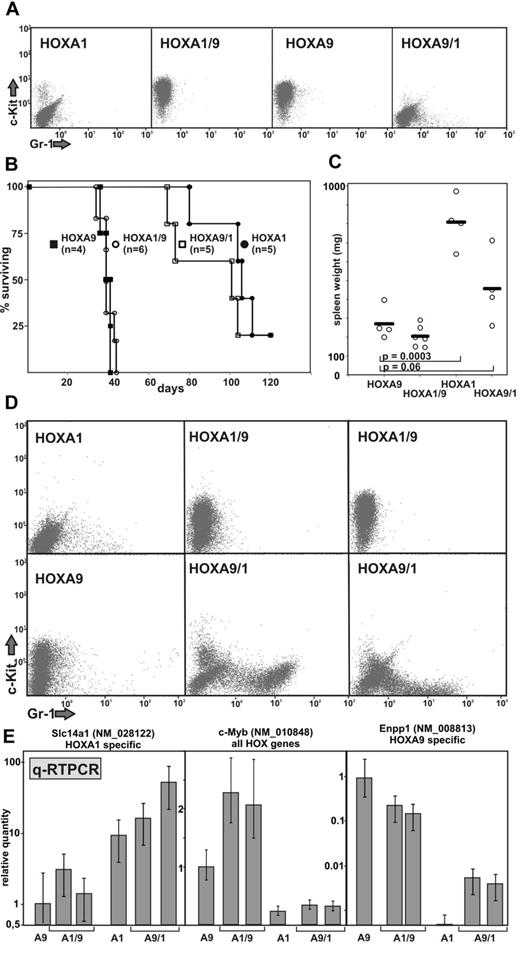
A good system to assess the paralog-specific properties of HOX genes is the induction of acute leukemia. As previously shown,34 wild-type HOXA1 causes leukemia with a long latency and reduced penetrance, whereas HOXA9 induces disease very rapidly and efficiently. Both proteins, however, need coexpression of Meis1 for efficient leukemogenesis. For that reason, hematopoietic precursor cells were cotransduced with Meis1 and the swap constructs or the parental HOX genes as control (Figure 3). Surface marker analysis of the double-transduced cells corroborated the homeodomain/phenotype correlation. HOXA9 as well as HOXA1/9 cells were arrested at an earlier stage of differentiation displaying higher c-Kit levels than the HOXA1 or HOXA9/1 populations (Figure 3A). For leukemia induction, these cells were transplanted into syngenic, lethally irradiated recipients together with nontransduced bone marrow as radioprotectant (Figure 3B). Animals were monitored on a daily basis; and on first signs of overt disease (cachexia, ruffled fur, altered behavior, abnormal posture), euthanasia and a thorough post mortem analysis were performed. Spleen weight was recorded, and permanent cell lines were generated from the spleen of the recipients as a definite proof for the presence of leukemia. Again, all aspects of in vivo leukemogenesis in these experiments were determined nearly exclusively by the nature of the homeodomain. Equipping the N-terminus of HOXA1 with a HOXA9 homeodomain decreased latency of the resulting leukemia dramatically. In contrast, a HOXA9 amino terminus with a HOXA1 homeodomain protracted the disease course. Another characteristic difference between HOXA1- and HOXA9-induced leukemia was the spleen weight of recipient animals (Figure 3C). “HOXA1 leukemia” was generally associated with a more pronounced splenomegaly compared with HOXA9-initiated disease. This feature was also largely controlled by the homeodomain. However, similar to the gene expression experiments, HOXA1/9 was a better “mimic” of HOXA9 also in vivo, whereas the reciprocal fusion lead to a less complete conversion. This was also underlined by the phenotype of cell lines established directly from leukemic spleen (Figure 3D). An “A9-type” DNA binding domain led to the development of more stem cell-like features with a high level of c-Kit and no evidence for the presence of the differentiation marker Gr-1. The presence of a HOXA1-type homeobox area caused the opposite effect that was more pronounced in HOXA9/1 cells. Expression of HOX-sentinel genes in these cell lines followed the same pattern as observed in vitro with the interesting exception that myb expression seemed to be proportional to disease latency (Figure 3E).
The phenotype of HOX-induced leukemia is governed by the homeodomain. (A) Surface immunophenotype of cells cotransduced with Meis1 and HOX or a HOX swap as indicated. Staining was done for the stem cell and precursor cell marker c-Kit (CD117-PE) and for Gr-1 (Gr1-FITC) a protein associated with myeloid differentiation. (B) Kaplan-Meier survival blot of recipient animals transplanted with the cells characterized in panel A. Irradiated recipients received the graft at day 0. Animals were killed according to institutional regulations at first signs of overt disease. The experiment was terminated after 120 days, with 1 animal of the HOXA1 and HOXA9/1 cohorts still surviving. n = number of animals per group. (C) Spleen weight of leukemic animals. Individual data points and average values are given. P values were calculated for a Student t distribution. (D) Representative example of leukemia cell immunophenotypes. Transformed cells were regrown from the spleen of diseased animals and analyzed by FACS for c-Kit and Gr-1 markers. (E) Quantitative RT-PCR of HOX-sentinel genes in leukemia samples. RNA was isolated from the explanted cell lines, and HOX-specific gene expression was examined by quantitative RT-PCR. Each bar represents an individual cell line. Data are mean ± SD of triplicate PCR experiments.
The phenotype of HOX-induced leukemia is governed by the homeodomain. (A) Surface immunophenotype of cells cotransduced with Meis1 and HOX or a HOX swap as indicated. Staining was done for the stem cell and precursor cell marker c-Kit (CD117-PE) and for Gr-1 (Gr1-FITC) a protein associated with myeloid differentiation. (B) Kaplan-Meier survival blot of recipient animals transplanted with the cells characterized in panel A. Irradiated recipients received the graft at day 0. Animals were killed according to institutional regulations at first signs of overt disease. The experiment was terminated after 120 days, with 1 animal of the HOXA1 and HOXA9/1 cohorts still surviving. n = number of animals per group. (C) Spleen weight of leukemic animals. Individual data points and average values are given. P values were calculated for a Student t distribution. (D) Representative example of leukemia cell immunophenotypes. Transformed cells were regrown from the spleen of diseased animals and analyzed by FACS for c-Kit and Gr-1 markers. (E) Quantitative RT-PCR of HOX-sentinel genes in leukemia samples. RNA was isolated from the explanted cell lines, and HOX-specific gene expression was examined by quantitative RT-PCR. Each bar represents an individual cell line. Data are mean ± SD of triplicate PCR experiments.
To test the correlation of the homeodomain and the phenotype of HOX-transformed cells in a different model, an additional HOXA9/13 swap was tested (supplemental Figure 2). As we have shown previously,34 HOXA13 directs differentiation of hematopoietic cells toward monocytes and macrophages, whereas HOXA9-transformed cells give rise to mainly mixed precursor/granulocyte populations. This particular phenotype of HOXA13-transduced cells manifests itself in the appearance of “mobile” cells that migrate through methylcellulose during replating assays. In addition, HOXA13 cells have the classic monocyte/macrophage morphology and display higher levels of Gr-1 and CD14 surface markers compared with HOXA9 transformed populations. Indeed, all of these features were completely recapitulated by the HOXA9/13 chimera corroborating the importance of the homeodomain as specificity factor.
Several features within the larger homeodomain region cooperate to determine HOX specificity
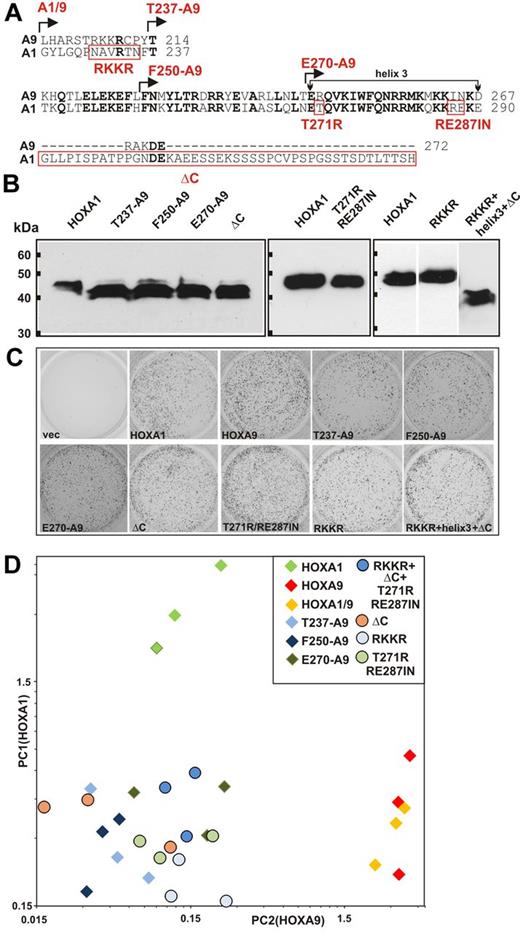
Because of the particular design, the HOXA9/1 fusion recreates the original HOXA9 basic tetrad upstream of the A1 core-homeobox. In normal HOXA1, this motif is reduced to a single arginine. A structure-function analysis (Figure 4) was performed to elucidate whether this vestigial feature of HOXA9 might be the molecular reason for the less complete conversion of the HOXA9/1 chimera compared with the reciprocal construct. For this purpose, 2 sets of HOXA1 mutants were created. First, additional HOXA1/9 swap derivatives were cloned with the junctions between HOXA1 and HOXA9 sequences moved incrementally toward the C-terminus (Figure 4A). In addition, 4 further mutants were tested that introduce single aspects of the HOXA9 hoemeodomain into the context of HOXA1: (1) the arginine preceding the core homeobox was changed to the more extended basic feature of HOXA9 (RKKR); (2) charged amino acids of recognition helix 3 in HOXA1 were converted to those present at the same position in HOXA9 (T271R/RE287IN); (3) the long C-terminal extension of HOXA1 following the homeobox that has no counterpart in HOXA9 was removed (ΔC); and (4) a combination of the 3 aforementioned features. After confirmation of correct expression (Figure 4B), hematopoietic precursor cells were transduced with the respective mutants. Each construct was capable of immortalizing hematopoietic progenitors in replating assays (Figure 4C), indicating that the altered homeodomain regions were still capable of inducing at least the minimal set of genes necessary for in vitro transformation. Three independent cell lines were generated for each mutant. The degree of “HOXA9-ness” was quantified by a modified principal component analysis. The expression levels of the top 5 genes most characteristic for HOXA1 (Scin, Gzmb, Col18a1, Crhbp, and Ptprz1) or HOXA9 (Gpx8, Ly6f, Airn, Cyp26a1, and Ccl8) were measured by quantitative RT-PCR for each line. Relative expression values for HOXA1 and HOXA9 specific genes were calculated and added to yield a HOXA1 (PC1) and a HOXA9 (PC2) component (Figure 4D). All 3 major features within the homeodomain region (basic tetrad, helix 3, short C-terminal extension) were absolutely necessary to induce a full HOXA9-type expression pattern. Fusing only helix3 and/or the short HOXA9 C-terminus to HOXA1 (clones T237-A9; F250-A9; E270-A9) led to a loss of HOXA9-specific gene expression. The increased HOXA1 portion, though, did not yet bring back a complete HOXA1 specific pattern. This was only possible with complete HOXA1 where the reduced basic patch, helix3 and the C-terminal extension cooperated. The individual conversion of each of these features to HOXA9 identity (clones RKKR, T271R/RE287IN, ΔC) caused a loss of HOXA1 specific transcripts but did not rescue a “HOXA9-type” expression pattern. Even a combined conversion of these 3 determinants could not rescue the HOXA9 “genotype” indicating that the determinants interrogated by our mutants are necessary but not sufficient to determine the specificity of the DNA binding domain. Therefore, the recognition of paralog-specific genes beyond a shared “transforming” target set is a consequence of a collaboration of multiple features within the extended homeodomain region. This gives a probable explanation why the reciprocal HOXA9/1 fusion did not completely mimic HOXA1.
Multiple features in and around the homeodomain cooperate to determine specificity. (A) Alignment of the homeodomain area of HOXA9 and HOXA1 and structure of mutants. Various portions of the HOXA9 homeodomain region were swapped for the corresponding sequences of HOXA1 at homologous positions. The junction sites of the resulting mutants are indicated at the top of the sequence by arrows. A1/9 corresponds to the original swap described in Figure 1. Single features of HOXA9 introduced into the context of HOXA1 are marked by red boxes and labeled below the amino acid sequence. (B) Expression of HOXA1 mutants. All constructs carried an HA tag. Comparable expression is demonstrated by anti-HA Western blot. (C) Activity of HOXA1 mutants in replating assays. The figure depicts third round colonies in methylcellulose obtained with primary hematopoietic progenitors transduced with individual mutants as described in panel A. Three independent transductions were done for each construct and the figure shows a typical result. (D) Principal component analysis of HOXA1 and HOXA9 specific gene expression. Three independent cell lines were derived for each HOX mutant by transduction of primary hematopoietic cells. Expression of the 10 most discriminatory genes that distinguish cells transformed by HOXA1 from those expressing HOXA9 (A1: Scin, Gzmb, Col18a1, Crhbp, and Ptprz1; A9: Gpx8, Ly6f, Airn, Cyp26a1, and Ccl8) was determined by quantitative RT-PCR relative to actin. Normalized expression levels across all samples were used to build a principal component (PC1) indicative for HOXA1 gene expression and a principal component (PC2) characteristic for HOXA9. Diamonds represent swap mutants; circles, HOXA1 mutants with single features changed to HOXA9; labeled according to the nomenclature in panel A.
Multiple features in and around the homeodomain cooperate to determine specificity. (A) Alignment of the homeodomain area of HOXA9 and HOXA1 and structure of mutants. Various portions of the HOXA9 homeodomain region were swapped for the corresponding sequences of HOXA1 at homologous positions. The junction sites of the resulting mutants are indicated at the top of the sequence by arrows. A1/9 corresponds to the original swap described in Figure 1. Single features of HOXA9 introduced into the context of HOXA1 are marked by red boxes and labeled below the amino acid sequence. (B) Expression of HOXA1 mutants. All constructs carried an HA tag. Comparable expression is demonstrated by anti-HA Western blot. (C) Activity of HOXA1 mutants in replating assays. The figure depicts third round colonies in methylcellulose obtained with primary hematopoietic progenitors transduced with individual mutants as described in panel A. Three independent transductions were done for each construct and the figure shows a typical result. (D) Principal component analysis of HOXA1 and HOXA9 specific gene expression. Three independent cell lines were derived for each HOX mutant by transduction of primary hematopoietic cells. Expression of the 10 most discriminatory genes that distinguish cells transformed by HOXA1 from those expressing HOXA9 (A1: Scin, Gzmb, Col18a1, Crhbp, and Ptprz1; A9: Gpx8, Ly6f, Airn, Cyp26a1, and Ccl8) was determined by quantitative RT-PCR relative to actin. Normalized expression levels across all samples were used to build a principal component (PC1) indicative for HOXA1 gene expression and a principal component (PC2) characteristic for HOXA9. Diamonds represent swap mutants; circles, HOXA1 mutants with single features changed to HOXA9; labeled according to the nomenclature in panel A.
Essential features within the HOX N-termini
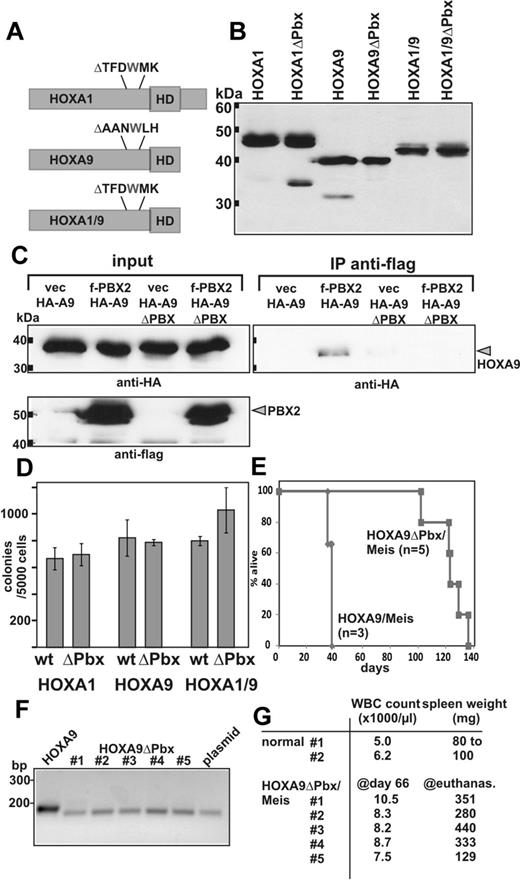
Although there is no significant sequence homology between the N-termini of HOXA1 and HOXA9, our results show that the 2 domains are functionally interchangeable. As a consequence, they must share features that, in combination with the respective homeobox, allow establishment of a paralog specific gene expression pattern. The only common denominator of both proteins described in literature is an interaction with PBX cofactors.22,24,42,43 Pbx2/Hoxa9 complexes have been detected in myeloid cells27 and functional cooperation has been demonstrated on DNA templates. Association of Hoxa1 with Pbx1 has been inferred indirectly from EMSA experiments where Hoxa1 and Pbx1 bind cooperatively if tethered to DNA by adjacent recognition sites. In both cases, a tryptophan-containing motif was absolutely essential for interaction with PBX. Therefore, we asked whether PBX binding is a common biologic activity of the HOXA1 and HOXA9 N-termini by testing PBX binding mutants (Figure 5). Derivatives of HOXA1, HOXA9, and HOXA1/9 were created that deleted the crucial tryptophan, including several flanking amino acids (Figure 5A). This manipulation did not alter protein stability as all constructs were expressed at levels comparable to the wild-type versions (Figure 5B). In coimmunoprecipitation experiments, the deletion of the W-motif completely prevented DNA-dependent binding of HOXA9 to PBX2 (Figure 5C). Under identical conditions, HOXA1 did not coprecipitate with PBX1 or PBX2, indicating that the binding affinity was below our detection limit. Still, as deducible from the literature, similar deletions abolish cooperative DNA binding with PBX44 and mimic a knockout phenotype of HOXA1 during mouse development.43 Interference with PBX binding had no detectable effect on in vitro transformation as tested in replating assays (Figure 5D). Colony numbers for all PBX binding mutants did not differ significantly from those obtained with the parental constructs corroborating similar results previously reported for HOXA9.32,33 In contrast, the PBX binding motif was essential for efficient induction of leukemia in transplantation assays (Figure 5E). Whereas animals transplanted with HOXA9/Meis died of disease after 5-6 weeks, recipients receiving a HOXA9ΔPBX/Meis graft were still alive after 10 weeks. The HOXA9ΔPBX transgene was detectable in these animals by PCR in genomic DNA from peripheral blood indicating engraftment (Figure 5F). Yet, the recipients showed only slightly elevated WBC counts at that time point (Figure 5G). Eventually, leukemia also developed in HOXA9ΔPBX/Meis-transplanted animals after a protracted latency of 14-19 weeks. From the spleens of all mice, HOXA9ΔPBX/Meis cells could be rederived for in vitro culture and the presence of the transgene was confirmed by PCR on genomic DNA (not shown).
Impact of PBX binding on transformation. (A) Deletion mutants removing the tryptophan and adjacent amino acids essential for PBX binding were developed as schematically depicted. Like the parental constructs, all deletion derivatives carried an N-terminal HA-epitope. (B) Anti-HA immunoblot testing the expression of PBX-deleted HOX proteins compared with the original version. (C) Coimmunoprecipitation of PBX2 with HOXA9 and HOXA9ΔPBX. Flag-tagged PBX2 or empty vector as control was coexpressed together with HA-labeled HOXA9 or the respective PBX binding site mutant. Immunoprecipitation was done with anti-flag antibodies and the precipitates were analyzed by anti-HA immunoblot for the presence of HOXA9 protein. As control, antiflag and anti-HA blots of the input are shown alongside. (D) Colony numbers obtained in replating assays performed with primary bone marrow cells transduced either with HOX or a HOXΔPBX construct as indicated. Data are mean ± SD of a biologic triplicate. (E) Kaplan-Meier survival plot of mice transplanted either with cells cotransduced with HOXA9/Meis (n = 3) or HOXA9ΔPBX/Meis (n = 5) as indicated. (F) Engraftment of HOXA9ΔPBX/Meis cells. Genomic DNA was isolated from a peripheral blood sample drawn at day 66 after transplantation from HOXA9ΔPBX/Meis recipients. The HOX transgene was detected by PCR with primers spanning the PBX deletion site. As controls, DNA from a HOXA9/Meis graft (lane HOXA9) and a plasmid containing HOXA9ΔPBX (lane plasmid) were used. Expected amplicon sizes are 190 bp for HOXA9 and 172 bp for HOXA9ΔPBX. (G) White blood cell (WBC) count at day 66 after transplantation in peripheral blood of the HOXA9ΔPBX/Meis recipients and 2 nontransplanted animals (control). Spleen weight of normal animals and of HOXA9ΔPBX/Meis recipients at day of death is listed in the right column.
Impact of PBX binding on transformation. (A) Deletion mutants removing the tryptophan and adjacent amino acids essential for PBX binding were developed as schematically depicted. Like the parental constructs, all deletion derivatives carried an N-terminal HA-epitope. (B) Anti-HA immunoblot testing the expression of PBX-deleted HOX proteins compared with the original version. (C) Coimmunoprecipitation of PBX2 with HOXA9 and HOXA9ΔPBX. Flag-tagged PBX2 or empty vector as control was coexpressed together with HA-labeled HOXA9 or the respective PBX binding site mutant. Immunoprecipitation was done with anti-flag antibodies and the precipitates were analyzed by anti-HA immunoblot for the presence of HOXA9 protein. As control, antiflag and anti-HA blots of the input are shown alongside. (D) Colony numbers obtained in replating assays performed with primary bone marrow cells transduced either with HOX or a HOXΔPBX construct as indicated. Data are mean ± SD of a biologic triplicate. (E) Kaplan-Meier survival plot of mice transplanted either with cells cotransduced with HOXA9/Meis (n = 3) or HOXA9ΔPBX/Meis (n = 5) as indicated. (F) Engraftment of HOXA9ΔPBX/Meis cells. Genomic DNA was isolated from a peripheral blood sample drawn at day 66 after transplantation from HOXA9ΔPBX/Meis recipients. The HOX transgene was detected by PCR with primers spanning the PBX deletion site. As controls, DNA from a HOXA9/Meis graft (lane HOXA9) and a plasmid containing HOXA9ΔPBX (lane plasmid) were used. Expected amplicon sizes are 190 bp for HOXA9 and 172 bp for HOXA9ΔPBX. (G) White blood cell (WBC) count at day 66 after transplantation in peripheral blood of the HOXA9ΔPBX/Meis recipients and 2 nontransplanted animals (control). Spleen weight of normal animals and of HOXA9ΔPBX/Meis recipients at day of death is listed in the right column.
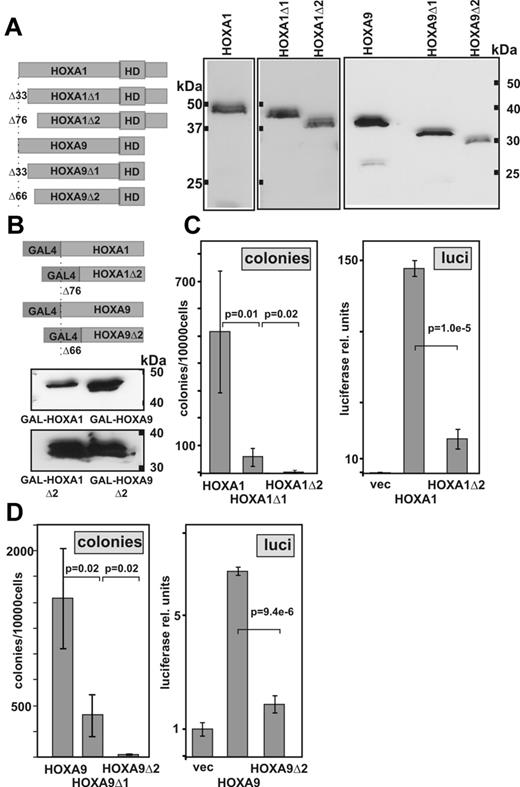
In contrast to the PBX binding motif, and corroborating previous results obtained for Hoxa9,17,45 the very N-terminus of both HOXA9 and HOXA1 was essential also for in vitro transformation (Figure 6). Deletion of the first 66 amino acids in HOXA9 or 76 amino acids in HOXA1 completely prohibited self-renewal of hematopoietic cells. In HOXA9, this region had been reported to be necessary to stabilize cooperative DNA binding of Meis1 and Hoxa9 on artificial DNA templates in vitro.26 However, no evidence for a similar interaction between Hoxa1 and Meis has been reported up to today. Hence, it seemed likely that this domain must have a second function beyond providing a Meis1 binding interface. HOX proteins are transcription factors and a transactivation domain residing in the amino terminus of HOXA9 has been identified in a previous publication.17 Thus, we tested the respective N-terminal sequences and the deletion mutants of HOXA1 and HOXA9 in a GAL4-based reporter assay. Indeed, in these experiments, both N-termini served as strong transcriptional activators. Transactivation and transformation were clearly correlated as deletion mutants that impaired colony formation also caused a significant loss in transactivation capacity.
A transactivation domain in the HOX N-terminus is essential for transformation. (A) Design and expression of HOX N-terminal deletion derivatives. Immunoblotting was done with an anti-HA antibody detecting an engineered epitope tag. (B) Structure and expression of GAL4-HOX constructs. To avoid interference with GAL4-based DNA binding, only the respective HOX N-terminus without the homeodomain was fused to GAL4. Western blotting was performed in lysates of 293T cells transfected with the respective construct as indicated. An anti-GAL4 antibody was used for detection. (C) Structure-function correlation for N-terminal HOXA1 mutants. Left panel: Transforming activity of HOXA1 deletion clones as determined in replating assays. Data are mean ± SD of third round colony numbers representing 3 biologic replicates. P values were determined by Student t test. Right panel: Transactivation by corresponding GAL4-HOX fusions. REH cells were electroporated with 0.1 μg of a standard GAL4-SV40 minimal promoter vector (pGL3) and 0.9 μg of a GAL4-HOX expression plasmid. Luciferase values are given as mean ± SD of triplicates. Background values achieved with empty expression vector were set to 1 unit. (D) Transformation and transactivation determined for HOXA9 derivatives in analogy to the experiments shown in panel C.
A transactivation domain in the HOX N-terminus is essential for transformation. (A) Design and expression of HOX N-terminal deletion derivatives. Immunoblotting was done with an anti-HA antibody detecting an engineered epitope tag. (B) Structure and expression of GAL4-HOX constructs. To avoid interference with GAL4-based DNA binding, only the respective HOX N-terminus without the homeodomain was fused to GAL4. Western blotting was performed in lysates of 293T cells transfected with the respective construct as indicated. An anti-GAL4 antibody was used for detection. (C) Structure-function correlation for N-terminal HOXA1 mutants. Left panel: Transforming activity of HOXA1 deletion clones as determined in replating assays. Data are mean ± SD of third round colony numbers representing 3 biologic replicates. P values were determined by Student t test. Right panel: Transactivation by corresponding GAL4-HOX fusions. REH cells were electroporated with 0.1 μg of a standard GAL4-SV40 minimal promoter vector (pGL3) and 0.9 μg of a GAL4-HOX expression plasmid. Luciferase values are given as mean ± SD of triplicates. Background values achieved with empty expression vector were set to 1 unit. (D) Transformation and transactivation determined for HOXA9 derivatives in analogy to the experiments shown in panel C.
Discussion
Here we report that HOX specific phenotypes in transformed hematopoietic cells are largely controlled by the identity of the HOX DNA binding module (ie, the core homeobox and adjacent sequences). In contrast, the very divergent N-termini of HOXA1 and HOXA9 could be exchanged without significant consequences.
The problem of HOX target specificity is not completely understood. Most studies tackling this question have been done in Drosophila. Similar to our results early publications reported that replacing the homeodomain of fly Ultrabithorax (Ubx) with that of Antennapedia (Antp) converts Ubx to an Antp-like molecule.46 Gehring et al concluded that the homeodomain and neighboring sequences “almost entirely determine its functional specificity” in fly.47 In the meantime, however, the emerging picture is more complex, and it appears as if there are different classes of target genes. Some of those are recognized by the DNA binding region of the HOX protein alone, whereas others require the input of extradenticle/PBX or other cofactors. (For a comprehensive review of this problem see Mann et al.48 ) The fruit-fly genome encodes only 1 PBX homolog (Extradenticle) and 1 MEIS-related protein (Homothorax) that interact with 8 Drosophila Hox proteins. This restricts combinatorial possibilities compared with the 39 HOX, 4 PBX, and 5 MEIS/PKNOX genes of mammals. Therefore, we expected that cofactor binding patterns would be distinct for different HOX proteins and thus contribute to paralog specific gene expression. Surprisingly, however, specificity was mostly determined by the homeodomain region. This predominance of the DNA binding motif for target gene recognition implies that the N-termini of the model proteins HOXA1 and HOXA9 must be able to fulfill an identical function. Targeting the underlying biologic mechanism may represent an efficient strategy for an “anti-HOX” therapeutic intervention. PBX binding seems to be part of this common denominator. Both HOXA1 and HOXA9 contain an evolutionary conserved tryptophan residue that has been shown in crystal structures to insert into a hydrophobic binding pocket of PBX.40 In addition, functional assays indicated that PBX increases DNA binding affinity of mammalian HOX proteins.22,24,25,27 The importance of PBX for progenitor immortalization in replating assays is not yet completely clear. Whereas Schnabel et al describe that deletion of the hexapeptide motif in HOXA9 impairs its activity in replating assays,17 in our hands the PBX-interaction motif is dispensable for this effect, which goes along with previous findings by the Sauvageau and Kamps laboratories.29,32 In addition, oncogenic NUP98-HOXA9 fusion proteins were able to immortalize myeloid progenitors without the tryptophan motif responsible for interaction with PBX.33 In contrast to the in vitro replating studies in our hands, a loss of PBX binding in HOXA9 significantly impaired in vivo leukemic activity. This is reminiscent of a different yet related set of experiments49 that demonstrated that Hoxa9 critically needs Meis1 for leukemia induction but not for replating. Taking both results together, this suggests that the role of Meis1 in promoting formation of leukemic stem cells also involves gene regulation by Hoxa9-Pbx complexes.
The essential N-terminal transactivation domain may be another promising target to impede HOX activity. A deletion of the transactivation domain is accompanied by a loss of all transformation. In absolute numbers, HOXA1 seemed to be a stronger activator than HOXA9 despite its weaker oncogenic activity; however, this may be the result of the artificial environment of the GAL4-based reporter system. A previous report strongly corroborates the interdependence of transactivation and transformation.17 It has been shown that the VP16 transactivator domain can replace the N-terminus of HOXA9 without a deleterious effect on transforming efficiency. Transactivation does not seem to be a simple consequence of Meis1 binding as anterior HOX proteins HOXA1 to HOXA7 do not directly interact with MEIS.26 Still, HOXA1 collaborates with MEIS1 to induce leukemia.34 It may be possible that MEIS is recruited to HOXA1 through PBX/MEIS dimers that have been reported.50 Alternatively, the cooperation of HOX and MEIS may occur at the genetic level. MEIS has its own set of targets that can be activated in the absence of HOX proteins. These include genes, such as cyclinD3, which are involved in control of self-renewal capacity and proliferation.51 In addition, MEIS alone without any additional HOX input can induce leukemia if the transactivation capacity of the protein is “boosted” by fusing it to a VP16 transactivation domain,45,51 and this phenomenon was accompanied by an up-regulation of endogenous HoxA genes.52
In conclusion, it appears as if transformation of hematopoietic cells by HOX proteins, a process much less complex than embryonic development, can be governed by a sort of “simplified” HOX code where intrinsic specificity conferred by the homeodomain is sufficient. This has consequences for a strategy to develop therapeutics for a treatment of HOX-based leukemia. If all HOX proteins more or less depend on the same biologic mechanism to activate their targets, it may be possible to find inhibitors that aim at these oncoproteins in general instead of having to deal with each HOX member individually.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Renate Zimmermann for technical assistance and Sebastian Buhl for help in early stages of this work.
This work was supported by Deutsche Forschungsgemeinschaft (research funding; grant SL27/6-3; R.K.S.).
Authorship
Contribution: C.B., E.M., M.-P.G.-C., and R.K.S. performed and analyzed experiments; and R.K.S. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robert K. Slany, Genetics, University Erlangen, Erwin Rommel Strasse 3, 91058 Erlangen, Germany; e-mail: rslany@biologie.uni-erlangen.de.