In the study reported in this issue of Blood by Leung et al, detectable minimal residual disease (MRD) before hematopoietic stem cell transplantation (HSCT) was found to be prognostic for outcome, but did not prevent cure for children with acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML).1
The leukemic burden in blood and bone marrow can be sensitively quantified by various methods, most commonly by flow cytometry and PCR molecular amplification.2,3 MRD detection was first standardized and validated in the setting of Philadelphia-chromosome positive chronic myelogenous leukemia, where molecular detection of the BCR/ABL transcript is not only prognostic but also an essential therapeutic end point.4 MRD monitoring has subsequently been applied to other hematologic malignancies, including ALL and AML.2,3 The prognostic value of MRD detection is indisputable, with improved sensitivity in comparison to conventional histopathologic response criteria, and MRD is increasingly used to augment microscopy in guiding treatment decisions. MRD persistence or recurrence confers high risk of relapse and death from leukemia and thus prompts treatment intensification, which in some cases includes HSCT. However, recent studies have demonstrated that pretransplantation MRD is the strongest predictor of posttransplantation outcome in children and adults with ALL and AML.5-8 Various levels of MRD have been shown to confer relatively greater risk of relapse (eg, > 10-4 by PCR, > 0.01% by flow cytometry). This has led to consideration of risk stratification and treatment intensification when such thresholds are exceeded,8 and certain centers attempt to achieve MRD reduction or eradication before proceeding to transplant. Notably, the provision of additional antileukemia therapy before HSCT is not without risk because disease progression and/or treatment complications might ultimately preclude transplantation. This has raised the question as to whether the level of MRD should be used to govern “go/no go” decisions in regard to transplantation.
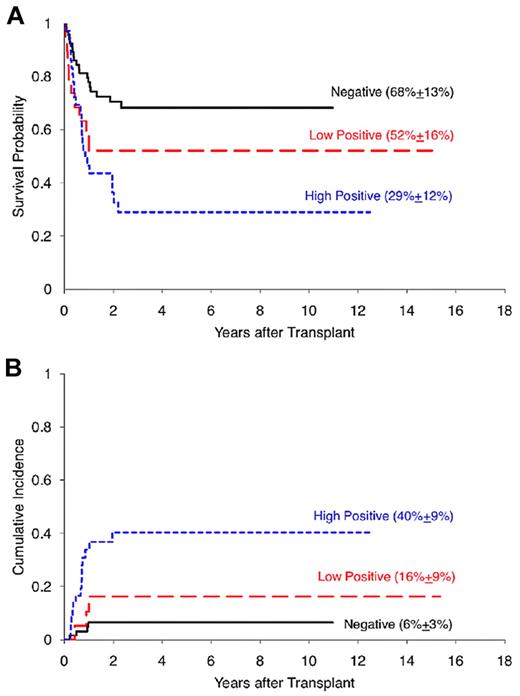
The study by Leung et al suggests that the mere presence of MRD is not an absolute obstacle to survival after allogeneic transplantation. They report posttransplantation outcomes in children with ALL (n = 64) and AML (n = 58) in relation to MRD levels determined by flow cytometry at the time of transplant. Higher MRD predicted poorer survival (P = .0019) and higher relapse (P = .0002) rates, and was an independent prognostic factor in a multivariate analysis (P = .0035). Children with acute leukemia and detectable MRD before transplant had survival rates of 29% ± 12% for patients with high levels of MRD and 52% ± 16% for those with lower levels of MRD, while those without detectable MRD had a relapse risk of 6% ± 3% (see figure). Moreover, patients treated on contemporary transplant protocols had improved outcomes compared with historical results, and this improvement was seen even in the face of pretransplantation MRD. Five-year survivals were greater than 80% for cases without MRD and exceeded 50% for patients with MRD. This is in keeping with recent data suggesting that many improvements in transplant procedures (eg, targeted chemotherapy levels, immunosuppressive regimens, antiviral and antifungal therapies) have produced a steady improvement in transplant results.9
Posttransplantation outcomes stratified by pretransplantation MRD status. (A) Five-year overall survival probability. (B) Five-year cumulative incidence of relapse. MRD indicates minimal residual disease. See Figure 1 in the article by Leung et al beginning on page 468.
Posttransplantation outcomes stratified by pretransplantation MRD status. (A) Five-year overall survival probability. (B) Five-year cumulative incidence of relapse. MRD indicates minimal residual disease. See Figure 1 in the article by Leung et al beginning on page 468.
Allogeneic GVL effects have been well demonstrated to contribute to the curative potential of HSCT. Nonetheless, relapse remains the primary cause of treatment failure after transplant for hematologic malignancies.10 Importantly, the study by Leung et al teaches us that patients who are rendered MRD “negative” may still have disease below the current level of detection that can evade GVL activity, and that individuals who remain MRD “positive” nonetheless have a chance of cure with HSCT. Further reduction in relapse risk is likely to be facilitated by both the development of more sensitive MRD detection methodologies,11,12 as well as by novel strategies that can augment GVL effects or overcome leukemia-cell resistance to standard therapies.
Ultimately, randomized trials will inform whether pretransplantation MRD should used be as a therapeutic end point or simply a biomarker of disease status.10 However, in the meantime, with current methodologies it is essential to consider the level of MRD detection prognostic, but not “black and white”; in and of itself MRD should not be an excuse to abort a potentially curative allogeneic transplantation.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■
REFERENCES
National Institutes of Health