Abstract
Platelets from patients with diabetes are hyperreactive and demonstrate increased adhesiveness, aggregation, degranulation, and thrombus formation, processes that contribute to the accelerated development of vascular disease. Part of the problem seems to be dysregulated platelet Ca2+ signaling and the activation of calpains, which are Ca2+-activated proteases that result in the limited proteolysis of substrate proteins and subsequent alterations in signaling. In the present study, we report that the activation of μ- and m-calpain in patients with type 2 diabetes has profound effects on the platelet proteome and have identified septin-5 and the integrin-linked kinase (ILK) as novel calpain substrates. The calpain-dependent cleavage of septin-5 disturbed its association with syntaxin-4 and promoted the secretion of α-granule contents, including TGF-β and CCL5. Calpain was also released by platelets and cleaved CCL5 to generate a variant with enhanced activity. Calpain activation also disrupted the ILK-PINCH-Parvin complex and altered platelet adhesion and spreading. In diabetic mice, calpain inhibition reversed the effects of diabetes on platelet protein cleavage, decreased circulating CCL5 levels, reduced platelet-leukocyte aggregate formation, and improved platelet function. The results of the present study indicate that diabetes-induced platelet dysfunction is mediated largely by calpain activation and suggest that calpain inhibition may be an effective way of preserving platelet function and eventually decelerating atherothrombosis development.
Introduction
Calpains are Ca2+-regulated cysteine proteases that are responsible for the limited proteolysis of target proteins, resulting in their modification (eg, activation, inhibition, or altered sensitivity to intracellular signals) rather than in their degradation.1 In contrast to promiscuous degradative proteases, calpains cleave a relatively restricted set of protein substrates and use complex substrate recognition mechanisms involving primary and secondary structural features of target proteins to alter protein function and cellular signaling.2
Platelets express μ-calpain (calpain 1) and m-calpain (calpain 2); named for the micro- and millimolar Ca2+ concentrations, respectively, that are required to activate them in vitro.3 Although Ca2+ is the main regulator of calpain activity, protease activation does not simply occur in response to any stimuli that increases platelet Ca2+. Indeed, the regulation of proteolytic activity is complex and involves the association of calpain with a regulatory subunit, the endogenous inhibitor calpastatin,4 other interacting proteins,5 and binding to phospholipids.6,7 Genetic deletion of m-calpain results in embryonic lethality,8,9 but μ-calpain−/− mice are viable and demonstrate attenuated aggregation and clot retraction but normal bleeding times.10 Mechanistically, the latter effects were attributed to the tyrosine dephosphorylation of platelet proteins because μ-calpain−/− platelets exhibited high levels of tyrosine phosphatase 1B protein and activity. Moreover, either an inhibitor of the phosphatase or the generation of μ-calpain–tyrosine phosphatase 1B double-knockout mice has been shown to rescue the platelet defect.11 However, it is almost certain that the cleavage of a single phosphatase cannot account for the consequences of μ-calpain activation in platelets.
Pathophysiologically, the oxidative stress associated with type 2 diabetes results in the inactivation of the sarcoplasmic endoplasmic reticulum Ca2+ ATPase (SERCA-2) and a subsequent increase in platelet Ca2+ levels that is sufficient to activate calpain. This leads to the limited proteolysis of target proteins without eliciting full-blown platelet activation.12 Several platelet proteins are known to be calpain substrates, including spectrin, adducin, talin, CD31, the myosin light-chain kinase, and N-ethylmaleimide-sensitive-factor attachment receptor (SNARE) proteins such as SNAP-23 and vesicle-associated membrane protein 3 (VAMP-3).13 However, the spectrum of proteins that serve as pathophysiologically relevant targets of calpain in diabetic humans and their eventual role in the accelerated development of cardiovascular disease is unknown. Given that it is currently not possible to identify calpain targets on the basis of a specific recognition sequence, in the present study, we used a proteomic approach to identify novel calpain targets in diabetic platelets and to elucidate their role in platelet function. To determine which platelet proteins could be affected by calpain activation/inhibition in diabetic humans, we made use of the fact that peroxisome proliferator-activated receptor (PPAR)γ agonist therapy increases SERCA-2 expression, normalizes platelet Ca2+, attenuates calpain activation, and prevents substrate cleavage.12
Methods
For full details of all methods, please see supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Pioglitazone study population
A total of 13 patients with type 2 diabetes mellitus were used in this study (5 women and 8 men; mean age, 47 ± 5.4 years; age range, 30-70 years; see supplemental Tables 1 and 2 for patient characteristics and medications). Patients were treated with either pioglitazone 30 mg/d (Actos; Takeda) or placebo each for 12 weeks in a cross-sectional design. There was a 4-week washout period between the different treatments. The study protocol was approved by the ethics committee of the Technical University of Dresden (number EK 233112006), and all of the participants gave written informed consent in accordance with the Declaration of Helsinki. Nondiabetic, age-matched subjects (7 women and 8 men; mean age, 40.25 ± 6.5 years; age range, 23-50 years; glycated hemoglobin (HbA1c), 5.2% ± 0.6%; fasting plasma glucose, 5.1 ± 0.2mM) who had not taken any medication known to interfere with platelet aggregation for at least 10 days before the experiment served as the control group.
Diabetic study population
A total of 30 patients (16 women and 14 men; mean age, 47 ± 5.4 years; age range, 30-70 years) with type 2 diabetes mellitus attending the clinic for routine control visits were included in the study. Patients had to have HbA1c more than 7.4% and fasting plasma glucose of 8.2 ± 0.7mM. All patients were treated with insulin alone or in combination with metformin. Nondiabetic, age-matched subjects (12 women and 18 men; mean age, 42.4 ± 4.5 years; age range, 25-65 years; HbA1c, 5.2% ± 0.6%; fasting plasma glucose, 5.1 ± 0.2mM) who had not taken any medication known to interfere with platelet aggregation for at least 10 days before the experiment served as the control group. The study protocol was approved by the ethics committee of the Goethe University Hospital (number E 61/09 geschäfts number 86/09) and all of the participants gave written informed consent in accordance with the Declaration of Helsinki.
Animals
C57BL/6 mice (6-8 weeks of age) were from Charles River Laboratories. Floxed μ-calpain−/− mice (μ-calfl/fl) were generated by Taconic Artemis and bred with animals expressing the Cre deleter under the control of the PF4 promoter (The Jackson Laboratory) to generate mice lacking μ-calpain in platelets (PF4-μ-cal−/−). Mice were housed in conditions that conform to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication no. 85-23). Both the university animal care committees and the Federal Authorities for Animal Research, Regierungspräsidium Darmstadt (Hessen, Germany; #F28/17) approved the study.
Diabetes was induced with a single IP injection of streptozotocin (150 mg/kg) and animals were considered diabetic when fasting plasma glucose was more than 250 mg/dL. In some experiments, animals were treated by oral gavage with the calpain inhibitor A-705253 (30 mg/kg/d) for 12 days after 12 weeks of untreated diabetes.
Statistical analysis
Data are expressed as means ± SEM and statistical evaluation was performed using the Student t test for unpaired data or 1-way ANOVA followed by a Bonferroni t test where appropriate using Prism 5 software (GraphPad). P values < .05 were considered statistically significant.
Results
Proteomic analysis of platelets from patients with and without pioglitazone therapy
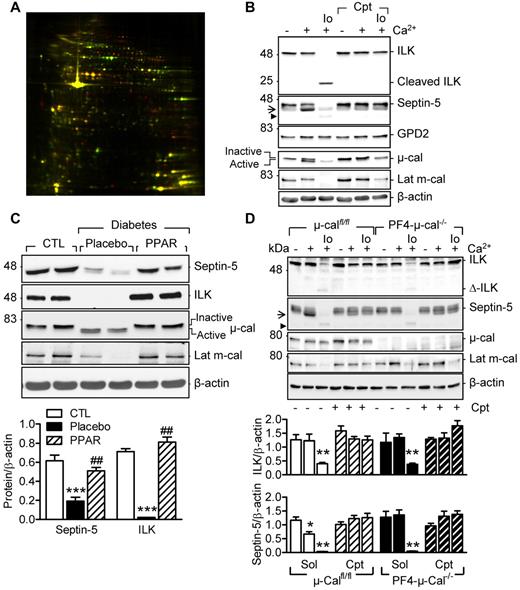
Platelets were obtained from diabetic subjects before and after 12 weeks of therapy with pioglitazone (30 mg/d). Pioglitazone therapy increased insulin sensitivity (supplemental Table 1) and had profound effects on the platelet proteome (Figure 1A, supplemental Figure 1, supplemental Table 3). More than half of the differentially expressed protein spots identified were known calpain substrates (eg, talin-1 and filamin A)13 and could be classified as cytoskeletal proteins and signaling molecules involved in metabolism and vesicle/secretory trafficking. Some of the proteins identified (eg, septin-5, the integrin-linked kinase [ILK] and glycerol-3-phosphate dehydrogenase 2 (GPD2) have not been previously linked with calpain.
Characterization of new calpain substrates in human and murine platelets. (A) Representative differential in-gel electrophoresis of platelet proteins from the same diabetic patient treated with placebo or pioglitazone (red spots indicate proteins down-regulated by pioglitazone; green spots, proteins that were up-regulated). (B) Representative blots showing the effect of Ca2+ (5mM) and ionomycin (Io; 1μM)–induced μ- and m-calpain activation on the levels of septin-5, ILK, and GPD2 in washed human platelets. (C) Levels of septin-5 and ILK in washed platelets from healthy donors (CTL) and patients with type 2 diabetes treated with placebo or pioglitazone (PPAR) (data shown from same patients). (D) Effect of μ- and m-calpain activation on septin-5 and ILK in platelets from μ-calfl/lfl and PF4-μ-cal−/− mice. Experiments were performed in the absence or in the presence of calpeptin (Cpt, 10μM). Arrow indicates the product after μ-calpain activation; arrowhead, product after m-calpain activation. Blots are representative of 5-6 additional experiments and graphs summarize data obtained in 8 subjects/animals per group. *P < .05; **P < .001; ***P < .001 versus healthy donors or untreated platelets; ###P < .001 versus placebo.
Characterization of new calpain substrates in human and murine platelets. (A) Representative differential in-gel electrophoresis of platelet proteins from the same diabetic patient treated with placebo or pioglitazone (red spots indicate proteins down-regulated by pioglitazone; green spots, proteins that were up-regulated). (B) Representative blots showing the effect of Ca2+ (5mM) and ionomycin (Io; 1μM)–induced μ- and m-calpain activation on the levels of septin-5, ILK, and GPD2 in washed human platelets. (C) Levels of septin-5 and ILK in washed platelets from healthy donors (CTL) and patients with type 2 diabetes treated with placebo or pioglitazone (PPAR) (data shown from same patients). (D) Effect of μ- and m-calpain activation on septin-5 and ILK in platelets from μ-calfl/lfl and PF4-μ-cal−/− mice. Experiments were performed in the absence or in the presence of calpeptin (Cpt, 10μM). Arrow indicates the product after μ-calpain activation; arrowhead, product after m-calpain activation. Blots are representative of 5-6 additional experiments and graphs summarize data obtained in 8 subjects/animals per group. *P < .05; **P < .001; ***P < .001 versus healthy donors or untreated platelets; ###P < .001 versus placebo.
To differentiate between calpain substrates and PPARγ-regulated genes, we determined the consequences of in vitro calpain activation on the levels of septin-5, ILK, and GPD2 in platelets from healthy subjects. Because platelet agonists alone are poor activators of the protease, calpains were activated via the Ca2+-sensing receptor by increasing the extracellular concentration of Ca2+ as described previously12 or using the combination of Ca2+ and ionomycin. The 2 approaches were also used to differentiate between μ- and m-calpain activation, both of which are expressed in platelets. In human platelets, extracellular Ca2+ was sufficient to stimulate the autolytic cleavage of μ-calpain and the limited degradation of septin-5 (Figure 1B). However, the combination of Ca2+ and ionomycin was required to activate m-calpain (decrease in latent m-calpain levels), which in turn resulted in the further degradation of septin-5 and ILK (Figure 1B). Because all of these changes were sensitive to the calpain inhibitor calpeptin, they could be attributed to calpain activation. GPD2 was unaffected by calpain activation, an observation that fits well with its classification as a PPARγ-regulated gene.14 We also confirmed that levels of septin-5 and ILK were reduced in diabetic versus healthy subjects and increased as a result of pioglitazone therapy, which also reduced μ- and m-calpain activation (Figure 1C).
Because calpain inhibitors are not able to differentiate between μ- and m-calpain, we studied the sensitivity of septin-5 and ILK to Ca2+-induced, calpain-mediated cleavage in mice lacking μ-calpain in platelets (PF4-μ-calpain−/− mice). The limited proteolysis of septin-5 was observed in Ca2+-stimulated platelets from μ-calfl/fl mice, but not their PF4-μ-cal−/− littermates (Figure 1D arrow). The combination of Ca2+ and ionomycin resulted in the further degradation of septin-5 (Figure 1D arrowhead) and was equally effective in μ-calfl/fl and PF4-μ-cal−/− mice. Conversely, the degradation of ILK was only observed in platelets treated with Ca2+ and ionomycin, and was unaffected by the platelet-specific down-regulation of μ-calpain.
Calpain-dependent cleavage of septin-5 increases α-granule secretion
Septin-5 is a cytosolic GTP-binding protein that inhibits platelet degranulation by binding the SNARE protein syntaxin-4.15 Septin-5 associated with syntaxin-4 in unstimulated platelets, but not after calpain activation with Ca2+ or the combination of Ca2+ and thrombin (Figure 2A). Conversely, calpain inhibition enhanced the syntaxin-4/septin-5 association. To determine the consequences of this interaction on platelet degranulation, we assayed the release of the dense granule marker serotonin and the α-granule protein TGF-β in the absence and presence of Ca2+ and thrombin. Serotonin release was stimulated by thrombin, but was not affected by calpain activation/inactivation (Figure 2B), whereas thrombin was able to stimulate TGF-β release only after Ca2+–induced calpain activation and not in the presence of the calpain inhibitor. Therefore, a calpain-sensitive process such as the cleavage of septin-5 seems to promote the release of α-granule contents actively.
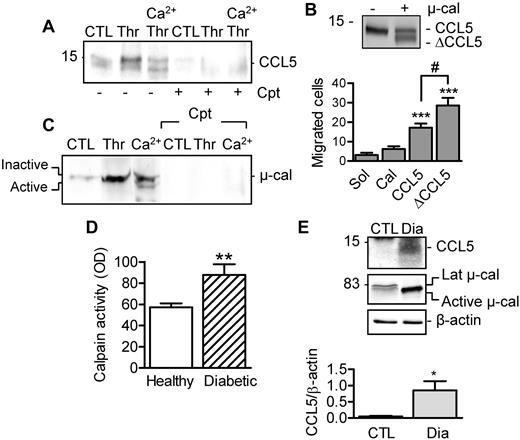
Calpain, septin-5, and platelet secretion. (A) Effect of calpain activation on the association of septin-5 with syntaxin-4 (Synt 4) in human platelets. (B) Levels of serotonin (5-HT) and TGF-β (ELISA) released from human platelets stimulated with either thrombin (Thr, 1 U/mL) or thrombin plus Ca2+ (to activate calpain). Experiments were performed in the absence and presence of calpeptin (Cpt, 10μM). (C) CCL5 levels in releasate from unstimulated and thrombin-stimulated platelets without and with calpain activation with Ca2+. Experiments were performed in the absence or in the presence of calpeptin (Cpt). (D) Effect of diabetes on CCL5 levels (ELISA) in plasma from healthy subjects and from patients with type 2 diabetes. (E) Immunostaining of CCL5 (red) released from platelets (β3 integrin [green]) onto endothelial cells (DAPI; white). Platelets were either left unstimulated (CTL) or treated with Ca2+ in the absence or in the presence of calpeptin (Cpt). Bars represent 20μM. The graphs summarize data obtained with 5-6 subjects per group. *P < .05, **P < .01, ***P < .001 versus CTL/Sol/Healthy.
Calpain, septin-5, and platelet secretion. (A) Effect of calpain activation on the association of septin-5 with syntaxin-4 (Synt 4) in human platelets. (B) Levels of serotonin (5-HT) and TGF-β (ELISA) released from human platelets stimulated with either thrombin (Thr, 1 U/mL) or thrombin plus Ca2+ (to activate calpain). Experiments were performed in the absence and presence of calpeptin (Cpt, 10μM). (C) CCL5 levels in releasate from unstimulated and thrombin-stimulated platelets without and with calpain activation with Ca2+. Experiments were performed in the absence or in the presence of calpeptin (Cpt). (D) Effect of diabetes on CCL5 levels (ELISA) in plasma from healthy subjects and from patients with type 2 diabetes. (E) Immunostaining of CCL5 (red) released from platelets (β3 integrin [green]) onto endothelial cells (DAPI; white). Platelets were either left unstimulated (CTL) or treated with Ca2+ in the absence or in the presence of calpeptin (Cpt). Bars represent 20μM. The graphs summarize data obtained with 5-6 subjects per group. *P < .05, **P < .01, ***P < .001 versus CTL/Sol/Healthy.
CCL5 (RANTES) is a chemokine stored in α-granules that can be deposited onto the endothelium, where it attracts monocytes and can promote the development of vascular disease.16 In contrast to TGF-β, small amounts of CCL5 were released from thrombin-stimulated platelets, but calpain activation increased CCL5 release significantly, whereas calpeptin inhibited it (Figure 2C). Moreover, circulating CCL5 was increased in plasma from diabetic subjects (Figure 2D), and more CCL5 was deposited by Ca2+-activated platelets onto endothelial cells (Figure 2E).
Cleavage of CCL5 by μ-calpain enhances chemotactic activity
Calpain activation in human platelets also resulted in CCL5 cleavage (Figure 3A). The latter phenomenon could be confirmed in vitro using the recombinant protein and resulted in the generation of a variant with enhanced monocyte chemotactic activity (Figure 3B). To identify the CCL5 variant generated by μ-calpain, platelet-derived CCL5 and recombinant CCL5 protein were exposed to μ-calpain in vitro and then subjected to mass spectrometry. These experiments identified the peptide YSSDTTPCCFAYIARPLPR (supplemental Figure 2), representing residues 3-21, suggesting that μ-calpain cleaves CCL5 at the 2 first N-terminal amino acids and at a C-terminal position.
Effect of calpain-mediated cleavage on CCL5 activity. (A) CCL5 release and cleavage after calpain activation in the absence and presence of calpeptin (Cpt, 10μM). (B) Cleavage of recombinant CCL5 by μ-calpain and chemotactic activity of full-length and calpain-cleaved CCL5 (ΔCCL5) on THP-1 cells in a Transwell chamber. (C) μ-calpain in releasates from resting platelets or platelets activated with thrombin alone or after stimulation with Ca2+ (5mM, 30 minutes) in the absence or in the presence of calpeptin (Cpt). (D) Calpain activity in plasma from healthy subjects and from patients with type 2 diabetes. (E) CCL5 and μ-calpain in circulating microparticles isolated from healthy subjects (CTL) and patients with type 2 diabetes (Dia). The graphs summarize data obtained with 5-6 subjects per group. *P < .05; **P < .01; ***P < .001 versus CTL/Sol; #P < .05 versus full-length CCL5.
Effect of calpain-mediated cleavage on CCL5 activity. (A) CCL5 release and cleavage after calpain activation in the absence and presence of calpeptin (Cpt, 10μM). (B) Cleavage of recombinant CCL5 by μ-calpain and chemotactic activity of full-length and calpain-cleaved CCL5 (ΔCCL5) on THP-1 cells in a Transwell chamber. (C) μ-calpain in releasates from resting platelets or platelets activated with thrombin alone or after stimulation with Ca2+ (5mM, 30 minutes) in the absence or in the presence of calpeptin (Cpt). (D) Calpain activity in plasma from healthy subjects and from patients with type 2 diabetes. (E) CCL5 and μ-calpain in circulating microparticles isolated from healthy subjects (CTL) and patients with type 2 diabetes (Dia). The graphs summarize data obtained with 5-6 subjects per group. *P < .05; **P < .01; ***P < .001 versus CTL/Sol; #P < .05 versus full-length CCL5.
Calpain-mediated CCL5 cleavage may take place either in α-granules or in the extracellular space after its release. We found that μ-calpain was secreted from thrombin-stimulated platelets (Figure 3C), a finding that fits well with a recent report that identified calpain in the platelet secretome.17 Indeed, calpain activity was measurable in plasma from healthy subjects and was increased significantly in plasma from diabetic patients (Figure 3D). Moreover, whereas only latent (inactive) μ-calpain was detected in circulating microparticles from nondiabetic subjects, active μ-calpain and high levels of CCL5 were detected in samples from diabetic subjects (Figure 3E).
μ-calpain activation disrupts the ILK-PINCH-Parvin complex and promotes ILK activation
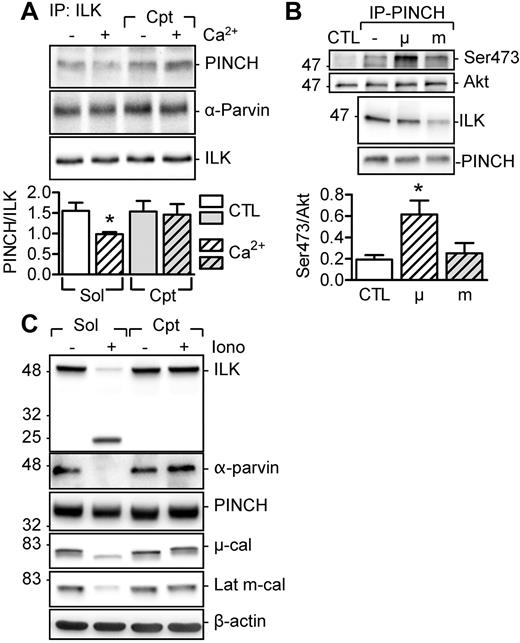
The second novel calpain substrate we identified was ILK. ILK forms the so-called ILK-PINCH-Parvin (IPP) complex, which is part of the focal adhesion complex and functions as a regulator of several signaling pathways.18 We found that PINCH and α-Parvin were coprecipitated with the ILK from unstimulated platelets, and the Ca2+-induced activation of μ-calpain resulted in the calpeptin-sensitive disassociation of PINCH from the kinase (Figure 4A). To determine the consequences of calpain activation on ILK activity, we assessed the ability of PINCH-ILK complexes to phosphorylate the ILK substrate Akt.19,20 The ILK that coprecipitated with PINCH from untreated human platelets and added to unstimulated platelet lysates elicited a moderate phosphorylation of Akt. However, when the coprecipitate was treated with μ-calpain to disassociate ILK from PINCH, Akt phosphorylation increased. Conversely, incubation of the immunoprecipitates with m-calpain resulted in the degradation of ILK and failed to elicit Akt phosphorylation (Figure 4B). In vitro stimulation of washed human platelets with ionomycin to activate both calpain isoforms led to the cleavage of ILK and α-Parvin, but did not affect the level of PINCH significantly, suggesting that, in addition to ILK, α-Parvin is also an m-calpain substrate (Figure 4C). The cleavage of both proteins was prevented by calpeptin. Therefore, μ-calpain activation promotes the release of active ILK from the IPP complex, whereas the activation of m-calpain degrades 2 of the IPP proteins.
Effect of calpain activation on ILK. (A) Effect of Ca2+ with or without calpeptin (Cpt, 10μM) on the association of PINCH and α-Parvin with ILK. (B) Effect of purified μ- and m-calpain on the ability of PINCH-ILK complexes to phosphorylate Akt (on Ser-473) in washed human platelets. (C) Consequences of calpain activation by ionomycin (Iono, 1μM) on the levels of ILK, α-Parvin, and PINCH in washed human platelets. Identical results were obtained in 4 additional experiments. The graphs summarize data obtained with 5-6 subjects per group. *P < .05 versus CTL/Sol.
Effect of calpain activation on ILK. (A) Effect of Ca2+ with or without calpeptin (Cpt, 10μM) on the association of PINCH and α-Parvin with ILK. (B) Effect of purified μ- and m-calpain on the ability of PINCH-ILK complexes to phosphorylate Akt (on Ser-473) in washed human platelets. (C) Consequences of calpain activation by ionomycin (Iono, 1μM) on the levels of ILK, α-Parvin, and PINCH in washed human platelets. Identical results were obtained in 4 additional experiments. The graphs summarize data obtained with 5-6 subjects per group. *P < .05 versus CTL/Sol.
Differential contribution of μ- and m-calpain to platelet adhesion and spreading
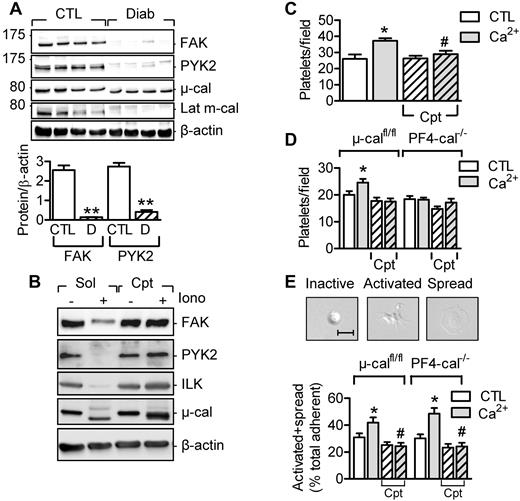
The IPP complex assembles in the cytoplasm and is recruited subsequently to focal adhesions.21 Therefore, we next focused on the role of calpain in regulating focal adhesion proteins and platelet adhesion and spreading. Indeed, 2 additional kinases linked to focal adhesion contacts, focal adhesion kinase (FAK) and proline-rich tyrosine kinase 2 (PYK2), were also attenuated in platelets from diabetic subjects (Figure 5A) and cleaved by calpain in vitro (Figure 5B). This is important because the proteolysis of focal adhesion proteins such as talin, paxillin (both are calpain substrates), and FAK is known to regulate focal adhesion dynamics and cell migration.22,23
Role of calpain in platelet adhesion and spreading. (A) Levels of FAK and PYK2 in washed platelets from healthy subjects (CTL) and from patients with type 2 diabetes (Diab/D) and activated μ- and m-calpain. (B) FAK, PYK2, and ILK are calpain substrates in vitro. Washed human platelets were treated with either solvent or ionomycin in the absence (Sol) and presence of calpeptin (Cpt, 10μM). (C) Adherence of human platelets pre-treated with and without Ca2+ in the absence or in the presence of calpeptin (Cpt) to fibronectin-coated slides. (D) Adherence of platelets from μ-calfl/fl or PF4-μ-Cal−/− mice pretreated with and without Ca2+ in the absence or in the presence of calpeptin (Cpt). (E) Effect of calpain inhibition on numbers of activated and spread platelets after stimulation of washed platelets from μ-calfl/fl and PF4-μ-Cal−/− mice with Ca2+ in the absence or in the presence of calpeptin (Cpt). The graphs summarize data obtained in platelets from 4-5 subjects or 6-9 animals per group. *P < .05; **P < .01 versus control; #P < .05 versus Ca2+-stimulated platelets without calpeptin.
Role of calpain in platelet adhesion and spreading. (A) Levels of FAK and PYK2 in washed platelets from healthy subjects (CTL) and from patients with type 2 diabetes (Diab/D) and activated μ- and m-calpain. (B) FAK, PYK2, and ILK are calpain substrates in vitro. Washed human platelets were treated with either solvent or ionomycin in the absence (Sol) and presence of calpeptin (Cpt, 10μM). (C) Adherence of human platelets pre-treated with and without Ca2+ in the absence or in the presence of calpeptin (Cpt) to fibronectin-coated slides. (D) Adherence of platelets from μ-calfl/fl or PF4-μ-Cal−/− mice pretreated with and without Ca2+ in the absence or in the presence of calpeptin (Cpt). (E) Effect of calpain inhibition on numbers of activated and spread platelets after stimulation of washed platelets from μ-calfl/fl and PF4-μ-Cal−/− mice with Ca2+ in the absence or in the presence of calpeptin (Cpt). The graphs summarize data obtained in platelets from 4-5 subjects or 6-9 animals per group. *P < .05; **P < .01 versus control; #P < .05 versus Ca2+-stimulated platelets without calpeptin.
In platelets from healthy subjects, Ca2+-induced calpain activation increased the number of platelets that adhered to fibronectin in a calpeptin-sensitive manner (Figure 5C). Similar responses were observed in platelets from μ-calfl/fl but not from PF4-μ-cal−/− mice (Figure 5D), suggesting a role for μ-calpain in platelet adhesion. Moreover, in platelets from healthy subjects, extracellular Ca2+ promoted lamellipodia formation and platelet spreading on fibronectin. The latter response was reduced in the presence of calpeptin in platelets from μ-calfl/fl and PF4-μ-cal−/− mice (Figure 5E). Therefore, m-calpain rather than μ-calpain seems to be activated during platelet spreading.
Calpain inhibition reverses diabetes-associated platelet hyperactivation
Our next step was to determine the effects of calpain inhibition on platelet function. In platelets from healthy subjects, the calpain inhibitors calpeptin and A-70525324 had moderate inhibitory effects on the thrombin-induced mobilization of Ca2+ and no significant effect on subsequent aggregation, but did attenuate clot retraction (supplemental Figure 3A-C). Both compounds prevented the Ca2+-induced cleavage of CD31, but the mechanisms of action were distinct: whereas calpeptin prevented the autolytic cleavage of μ-calpain, A705253 did not (supplemental Figure 3D).
Given that calpain is activated by redox stress and calpain inhibition had little effect on agonist-induced responses, we hypothesized that calpain treatment may preserve platelet function in a mouse model of diabetes. As with platelets from patients with type 2 diabetes, diabetes in wild-type mice (12 weeks after streptozotocin treatment) resulted in the activation of μ- and m-calpain and a reduction in levels of septin-5, ILK, FAK, and PYK2. Treating animals over 12 days with A-705253 (30 mg/kg/d orally) had no effect on blood glucose or bleeding times (supplemental Figure 4), but was sufficient to increase levels of latent μ- and m-calpain and increase the levels of their substrates to those observed in nondiabetic mice (Figure 6A).
Reversal of the diabetes-associated calpain overactivation by in vivo calpain inhibition. (A) ILK, septin-5, FAK, and PYK2 levels in platelets from healthy (CTL) and streptozotocin-induced diabetic mice (Dia) treated with vehicle or the calpain inhibitor A-705253 (A70). (B) CCL5 in microparticles isolated from the plasma of healthy (CTL) or diabetic (Dia) mice treated with vehicle or A-705253. (C) Effect of in vivo A-705253 treatment on thrombus size after FeCl3-induced injury of carotid arteries from healthy (top panel) and diabetic (bottom panel) mice. (D) Number of circulating platelet-leukocyte aggregates in healthy (CTL) and diabetic (Dia) mice treated with vehicle or A-705253. (E) Aggregation of platelets from healthy and diabetic mice treated with vehicle or A-705253. The graphs summarize data obtained in platelets from 6-9 animals per group. *P < .05; **P < .01 versus CTL; #P < .05; ##P < .01 versus vehicle-treated diabetic mice.
Reversal of the diabetes-associated calpain overactivation by in vivo calpain inhibition. (A) ILK, septin-5, FAK, and PYK2 levels in platelets from healthy (CTL) and streptozotocin-induced diabetic mice (Dia) treated with vehicle or the calpain inhibitor A-705253 (A70). (B) CCL5 in microparticles isolated from the plasma of healthy (CTL) or diabetic (Dia) mice treated with vehicle or A-705253. (C) Effect of in vivo A-705253 treatment on thrombus size after FeCl3-induced injury of carotid arteries from healthy (top panel) and diabetic (bottom panel) mice. (D) Number of circulating platelet-leukocyte aggregates in healthy (CTL) and diabetic (Dia) mice treated with vehicle or A-705253. (E) Aggregation of platelets from healthy and diabetic mice treated with vehicle or A-705253. The graphs summarize data obtained in platelets from 6-9 animals per group. *P < .05; **P < .01 versus CTL; #P < .05; ##P < .01 versus vehicle-treated diabetic mice.
As with the human subjects, diabetes increased levels of CCL5 detected in circulating murine microparticles (Figure 6B), and this response was reversed in animals treated with A-705253. CCL5 was also deposited onto endothelial cells in the carotid arteries of diabetic mice, a phenomenon reduced by the calpain inhibitor (supplemental Figure 5).
Diabetes also accelerated thrombus formation after FeCl3-induced carotid artery injury (the time to reach maximum size was 17.5 ± 1.2 minutes in healthy versus 7.5 ± 0.4 minutes in diabetic mice, n = 8 per group, P < .001; Figure 6C), potentiated the formation of leukocyte-platelet aggregates in vivo (Figure 6D), and enhanced thrombin-induced platelet aggregation in vitro (Figure 6E). Whereas thrombus formation in vivo in nondiabetic mice was unaffected by calpain inhibition, A-705253 reduced the maximum thrombus size and decelerated thrombus formation (time to maximum was increased from 7.5 ± 0.4 [n = 8] to 14.7 ± 2.4 minutes in the A-705253–treated diabetic mice [n = 7]; P < .05; Figure 6C), reversed the diabetes-induced formation of leukocyte-platelet aggregates in vivo (Figure 6D), and attenuated thrombin-induced platelet aggregation (Figure 6E).
Discussion
The results of the present study identified septin-5 and the ILK as novel calpain substrates in diabetic platelets and linked the proteolysis of these proteins to the increased secretion and modification of the α-granule protein CCL5 and to altered platelet adhesion and spreading. Moreover, it was possible to reproduce the findings using human platelets in a mouse model of diabetes and to prevent the in vivo proteolysis of the substrates identified using a calpain inhibitor. Calpain inhibition was also shown to reverse the hyperreactivity observed in diabetic platelets and the formation of platelet-leukocyte aggregates.
Over the past 10 years, it has become clear that platelets are not simply involved in aggregation and clot formation, but that they also play an active role in vascular homoeostasis, largely by delivering an impressive array of vasoactive substances. Little is known about the changes in platelets that lead to the enhanced deposition of chemokines such as stromal cell-derived factor-1,25 PF4, or CCL526 on the endothelial surface, which in turn promote the recruitment of circulating cells and can thus potentiate vascular disease development. Our data suggest that one of the major changes precipitating these effects is the activation of calpain. However, studying the relevance of these proteases in a human population is hampered by the fact that specific inhibitors are not available for human studies. Therefore, in the present study, we made use of a previously observed phenomenon: that PPARγ agonist therapy attenuates platelet Ca2+ levels and calpain activity.12 Our approach also meant that any positively regulated proteins could either be calpain substrates or proteins directly regulated by PPARγ. Interestingly, although we did detect one such protein (GPD2), a high percentage of the proteins sampled from the gels were previously characterized calpain substrates.
The first novel calpain substrate identified was septin-5 (also known as CDCrel-1 or PNUT-like), a SNARE-interacting protein previously identified as a Parkin substrate and implicated in the regulation of dopaminergic neurons.27,28 Interestingly, a neuron defect has been difficult to document in septin-5−/− mice, but platelets lacking the protein demonstrate enhanced aggregation and release stored granule products in response to stimuli that are under the threshold for normal platelets.15 Septin-5 has been localized to membrane areas surrounding platelet α-granules and binds syntaxin 4 to prevent rather than promote secretion.29 To date, it has been presumed that the phosphorylation of septin-5 in response to platelet agonists dissolves its association with syntaxin to promote granule product release29 ; however, our data suggest that this can also be achieved by the limited proteolysis of the protein by calpain. This implies that the loss of septin-5 function would result in enhanced secretion of platelet α-granule contents in the absence of overt platelet activation, which is exactly what was observed after calpain activation. More recently, different subpopulations of α-granules have been proposed to exist,30,31 and it will be interesting to determine whether calpain activation selectively results in the release of different α-granule subtypes.
In the present study, we focused on CCL5 as a marker of α-granule release, largely because of its described role in promoting the development of atherosclerosis.16,26 We found that calpain activation promoted the release of CCL5 and that the protein itself is a calpain substrate. The finding that μ-calpain was also secreted from thrombin-stimulated platelets, although initially unexpected, was consistent with our own finding that plasma calpain activity is much higher in samples from diabetic patients than from healthy subjects. It also fits well with a recent report that identified calpain in the platelet secretome.17 We also observed that the in vitro incubation of CCL5 with calpain resulted in the modification of CCL5 and the generation of a truncated variant (CCL5 3-21) that was more chemotactic for monocytes than the full-length protein. More than 19 variants of CCL5 are known to exist,32 and proteases such as dipeptidyl peptidase IV and cathepsin G can generate 3-68 and 4-68 variants of CCL5.33,34 Although the clinical significance of these variants is unclear, higher plasma levels of CCL5 (3-68) have been reported in patients with type 2 diabetes.32 However, the latter peptide was reported to show reduced rather than enhanced activity toward cells expressing the recombinant CCR5 receptor.33 This was not unexpected, because the N-terminus of many chemokines plays a critical role in the recognition and functional activation of cognate receptors. Given that the variant generated by calpain (3-21) enhanced monocyte adherence, it seems that calpain-mediated modifications of the C-terminus of CCL5 may more than compensate for the lower activity resulting from N-terminal truncation. It is unclear at the moment whether other platelet-derived products may be modified by extracellular calpain, but not all chemokines are calpain substrates, because MCP-1 could not be cleaved by calpain in an in vitro assay.
The second novel calpain substrate we focused on was ILK, which was first identified using a yeast 2-hybrid screen for β1-integrin–binding partners.35 ILK is part of the focal adhesion complex together with PINCH and Parvin,18 but even though kinase activity has been shown consistently with a recombinant protein,20,35 whether it acts as a kinase in vivo has proven controversial.36-38 Despite the controversy, a dual role for ILK in regulating integrin function and α-granule secretion can be extrapolated from studies in platelets from ILK-deficient mice. The latter demonstrate reduced aggregation, fibrinogen binding, and arterial thrombus formation, as well as a selective defect in α-granule but not dense-granule secretion.39 This phenotype fits well with that of the diabetic platelet. In addition, the results of the present study indicate that ILK is inactive when associated with PINCH and that μ-calpain initiates their dissociation and the subsequent phosphorylation of the ILK downstream target, Akt. A similar mechanism of action was recently proposed in a study looking at heart failure in zebrafish induced by the down-regulation of PINCH, which led to instability of ILK and the loss of Akt activity.40 Whereas our data suggest that μ-calpain is required for the activation of ILK, it seems that the activation of m-calpain results in the degradation of the kinase and prevents Akt phosphorylation.
Given the role of the IPP complex in the regulation of integrin signaling and the fact that the proteolysis of focal adhesion proteins such as talin, paxillin, FAK, and PYK2 is known to regulate focal adhesion dynamics and cell migration,22,23 it was logical to look at the consequences of calpain activation on platelet adhesion and spreading. We found that μ-calpain activation, which was also required for the activation of ILK, seems to regulate platelet adhesion (at least on fibronectin), whereas m- rather than μ-calpain regulates platelet spreading. The latter could be concluded only by comparing responses in platelets from μ-calfl/lfl and PF4-μ-cal−/− mice, because none of the currently available calpain inhibitors can differentiate between the μ- and m-calpain isoforms. Although the full spectrum of platelet kinases targeted by calpains remains to be elucidated, our data suggest that in vitro calpain activation and that associated with the development of diabetes must be linked with major changes in different intracellular signaling cascades. Indeed, ILK, PYK2, and FAK levels were almost undetectable in platelets from healthy subjects or mice after calpain activation in vitro or in diabetic platelets. However, normal levels of the kinases were observed in platelets from patients treated with a PPARγ agonist and diabetic animals treated with a calpain inhibitor.
It is well accepted that platelets contribute actively to the diabetes-associated accelerated development of atherothrombosis and enhanced platelet reactivity may account for the refractory response of diabetic patients to conventional antiplatelet therapy. The findings that calpain activity is elevated in platelets from diabetic subjects and that a calpain inhibitor reversed the diabetes-induced hyperactivation of platelets in mice suggest that Ca2+-activated proteases may be a promising therapeutic target for a better management of diabetes-associated platelet and vascular complications. Calpain inhibition may also underlie platelet activation in other conditions, because many of the proteins identified as calpain substrates in diabetic humans and mice (including ILK) were also reported to be altered in platelets from patients with acute coronary syndromes.41,42
Although in the present study, we found no significant effects on platelet number or function in otherwise healthy mice after 12 days of treatment, other studies indicate that the long-term treatment with A-705253 is well tolerated and can protect against retinal damage43 and penile nitrergic nerve and dysfunction in diabetic mice.44 Other calpain inhibitors attenuate angiotensin II–induced abdominal aortic aneurysms and atherosclerosis in LDL receptor–deficient mice45 and angiotensin II–induced endothelial dysfunction in rats and mice.46 The results of the present study, together with the studies showing a beneficial effect of calpain inhibition in vascular disease, highlight the potential of calpain inhibition for the treatment of the vascular complications of type 2 diabetes. However, future efforts should focus on the development of compounds devoid of the problems of calpain isoform selectivity and specificity versus other proteases,47 which have hampered the progression of calpain inhibitors into clinical trials.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Isabel Winter, Ingrid Kempter, and Katharina Engel-Herbig for technical assistance and Rory R. Koenen and Christian Weber (Ludwig Maximilians University, Munich, Germany) for help with CCL5 staining in carotid arteries.
This study was supported by the Deutsche Forschungsgemeinschaft (SFB 815/A16 and Z1 and the Exzellenzcluster 147 Cardio-Pulmonary System) and by the European Vascular Genomic Network, a network of excellence supported by the European Community's Sixth Framework Program (contract number LSHM-CT-2003-503254). A.E. was supported by a German Egyptian Research Long-term Scholarship funded by the Egyptian Ministry of Higher Education and Scientific Research and the German Academic Exchange Service. M.M. is a Senior Fellow of the British Heart Foundation.
Authorship
Contribution: V.R. codesigned the study, performed the experiments, and interpreted the results; J.I. and A.E. performed the experiments with human and murine platelets; F.P. performed the pioglitazone study and prepared the platelet samples; T.F. generated the PF4-μ-cal−/− mice; X.Y. and M.M. performed the differential in gel electrophoresis analysis of diabetic samples; K.B. provided the blood samples from characterized diabetic patients; H.H. performed the MS analysis of CCL5; and I.F. designed the study and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ingrid Fleming, PhD, Institute for Vascular Signalling, Centre for Molecular Medicine, Goethe University, Theodor-Stern-Kai 7, D-60590 Frankfurt am Main, Germany; e-mail: fleming@em.uni-frankfurt.de.
References
Author notes
V.R. and J.I. contributed equally to this work.

![Figure 2. Calpain, septin-5, and platelet secretion. (A) Effect of calpain activation on the association of septin-5 with syntaxin-4 (Synt 4) in human platelets. (B) Levels of serotonin (5-HT) and TGF-β (ELISA) released from human platelets stimulated with either thrombin (Thr, 1 U/mL) or thrombin plus Ca2+ (to activate calpain). Experiments were performed in the absence and presence of calpeptin (Cpt, 10μM). (C) CCL5 levels in releasate from unstimulated and thrombin-stimulated platelets without and with calpain activation with Ca2+. Experiments were performed in the absence or in the presence of calpeptin (Cpt). (D) Effect of diabetes on CCL5 levels (ELISA) in plasma from healthy subjects and from patients with type 2 diabetes. (E) Immunostaining of CCL5 (red) released from platelets (β3 integrin [green]) onto endothelial cells (DAPI; white). Platelets were either left unstimulated (CTL) or treated with Ca2+ in the absence or in the presence of calpeptin (Cpt). Bars represent 20μM. The graphs summarize data obtained with 5-6 subjects per group. *P < .05, **P < .01, ***P < .001 versus CTL/Sol/Healthy.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/120/2/10.1182_blood-2011-12-399980/4/m_zh89991294090002.jpeg?Expires=1763489881&Signature=ZoT4R7TJZDO0TzTFEXgg28iSpWtTPBbbCwQC0xFUL8dBFluPKNr7cer8Pg4WIPGFYh-HjlVJB4piFilEzgaSs5fcXgoyLrt1jJDlG9Dni2N5a4LxiMyZG1tBX7aSZyTH9g618UF-jv-XcVIsD4kNibnHI8I2D4IEb97BwztvtSNLZiszyIZrPsAzklCxMeYvtr9ecS0~fLFhP57x7888bGc1pSLFFHx90VuZzPFVossgtxh1pZIQrTAH99VZEh2gEAH5JpZ-rQkdUTrNhjzRjVIAcCy8o2qPk9WezHpVDs2X6LlUZEqYDI7yLSNULqduxyz8ovAJHGGArhd0OXxZPg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal