Abstract
Abstract 1070
Under conditions of pathological shear rate, von Willebrand Factor (vWF) undergoes conformational changes and self aggregation. We sought to visualize this phenomenon using a novel microfluidic model of stenosis and understand its role in thrombus formation in elevated shear rate environments. In severe stenosis, vWF experiences millisecond exposures to pathological wall shear rates (gw). Distinct from shear experiments that last many seconds, we deployed microfluidic devices for single-pass perfusion of whole blood or platelet free plasma (PFP) over fibrillar type 1 collagen (< 50 msec transit time) at pathological gw or spatial wall shear rate gradient (grad gw). Using fluorescent anti-vWF, long thick vWF fibers (>20 mm) bound to collagen were visualized at constant gw > 30,000 s−1 during perfusion of PFP, a process enhanced by EDTA. Rapid acceleration or deceleration of EDTA-PFP at grad gw = ± 5.5 × 105 to 4.3 × 107 s−1/cm did not promote vWF deposition when gw < 30,000. At 19,400 s−1, EDTA-blood perfusion resulted in rolling vWF-platelet nets, while blood perfusion (normal Ca2+) generated large vWF/platelet deposits that repeatedly embolized and were blocked by anti-GP1b or the aIIbβ3 inhibitor GR144053 and did not require shear gradients. Blood perfusion at venous shear rate (200 s−1) produced a stable platelet deposit that was a substrate for massive but unstable vWF-platelet aggregates when flow was increased to 7800 s−1. Supported by collagen and enhanced by platelet GP1b and aIIbβ3, vWF fiber formation occurred during acute exposures to pathological gw but did not require wall shear rate gradients.
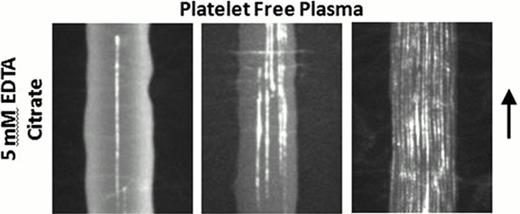
A, Platelet free citrated-plasma was treated with 1 μg/mL fluorescently labeled anti-vWF and 5 mM EDTA. The plasma samples were perfused over a collagen type 1 surface at local wall shear rates of 30,000, 62,400 and 125,000 s−1 from left to right. Long fibers of vWF (>20 μm) appeared at shear rates above ∼30,000 s−1, with more fibers appearing at higher shear rates. The bar indicates 15 μm.
A, Platelet free citrated-plasma was treated with 1 μg/mL fluorescently labeled anti-vWF and 5 mM EDTA. The plasma samples were perfused over a collagen type 1 surface at local wall shear rates of 30,000, 62,400 and 125,000 s−1 from left to right. Long fibers of vWF (>20 μm) appeared at shear rates above ∼30,000 s−1, with more fibers appearing at higher shear rates. The bar indicates 15 μm.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal