Abstract
Diffuse Large B-cell Lymphoma (DLBCL) is the most common lymphoma in the western world. Outcomes have improved with the standard initial therapy of rituximab and anthracyline based chemotherapy (immunochemotherapy). However, 20–40% of patients will either fail to achieve remission or relapse following initial immunochemotherapy. Most relapses occur early, and long-term outcome of relapsed/refractory patients is generally poor despite salvage regimens and stem cell transplant. Traditionally, studies for DLBCL have been evaluated using progression-free and/or overall survival. However, the event rate in DLBCL slows significantly approximately 12–18 months after diagnosis, and late events are often due to competing (non-DLBCL related) risks, especially in older patients. Here we examine outcome of DLBCL patients based on their event status at 12 months.
Newly diagnosed DLBCL patients were prospectively enrolled in the University of Iowa/Mayo Clinic Lymphoma SPORE Molecular Epidemiology Resource (MER). Clinical data were abstracted from medical records using a standard protocol. Patients were actively followed for events (progression, re-treatment, or death due to any cause) and overall survival (OS). Event-free status at 12 months after diagnosis (EFS12) was assessed as a dichotomous variable. Cause of death was determined by medical record and death certificate review using prospectively determined definitions. Expected survival was based on age and sex matched survival using the Minnesota population death rates. Confirmation cohorts were from a Lyon, France hospital based registry and NCCTG clinical trial N0489.
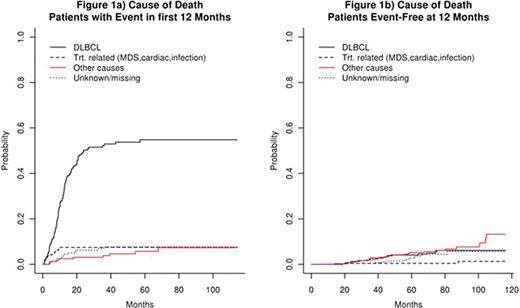
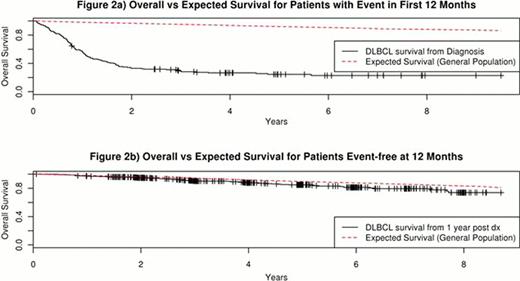
680 patients with newly diagnosed DLBCL and treated with rituximab + anthracycline based chemotherapy were enrolled in the MER from 2002 to 2009. The median age was 62 years (range 18–92). 53% were male. At a median follow-up of 59 months (range 8–116), 266 patients (39%) had an event and 188 patients (28%) died. 162 patients (24%) had an event in the first 12 months after diagnosis, comprising 60% of all events. Patients with an event in the first 12 months had poor survival with death almost exclusively due to disease (Figures 1a, 2a). In contrast, patients who were event-free at 12 months had comparable survival to the age and sex matched general population (Figure 2b) and were more likely to die of other causes than DLBCL (Figure 1b). Overall survival rates by EFS12 status were validated in patients from a French hospital-based registry (N=265) and the NCCTG N0489 clinical trial (N=87) with additional replication studies underway.
DLBCL patients who are event-free at 12 months after diagnosis have an excellent prognosis with an overall survival similar to that of an age- and sex- matched general population and are more likely to die of other causes than DLBCL. This finding has implications for clinical management, new clinical trials, and follow-up testing in this patient population. In contrast, patients with an event within the first 12 months after diagnosis have a poor prognosis with almost all deaths secondary to DLBCL. EFS status at 12 months identifies patients with poor outcomes due to disease. This can be utilized as an endpoint in biologic studies and clinical trials to address early treatment failures. EFS status at 12 months should be incorporated into prognostic models to identify high risk patients in newly diagnosed DLBCL.
No relevant conflicts of interest to declare.

This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal