Abstract
Abstract 1617
Romidepsin is a selective class 1 histone deacetylase inhibitor approved by the FDA for patients with cutaneous T-cell lymphoma and PTCL who have received at least 1 prior therapy. In recurrent/refractory PTCL, it has been evaluated as a single agent in 2 phase II studies with overall response rates of 25–38% (Piekarz, Blood 2011;117:5827; Coiffier, J Clin Oncol 2012;30:631). Toxicity was mainly hematologic and digestive. The aim of the present study was to evaluate the safety, tolerability and efficacy of different doses of romidepsin in association with CHOP in patients with previously untreated PTCL.
Patients with biopsy-proven PTCL were planned to receive 8 cycles of CHOP (cyclophosphamide 750 mg/m2 day 1, doxorubicin 50 mg/m2 day 1, vincristine 1,4 mg/m2 day 1, prednisone 40 mg/m2 days 1 – 5) in association with varying doses of romidepsin. Based on pharmacokinetic data and results of previous phase II studies, the starting dose of 10 mg/m2 on days 1 & 8 was chosen. The dose-variation scheme follows a traditional “3+3” design. Dose-limiting toxicity (DLT) were considered during the first 2 cycles.
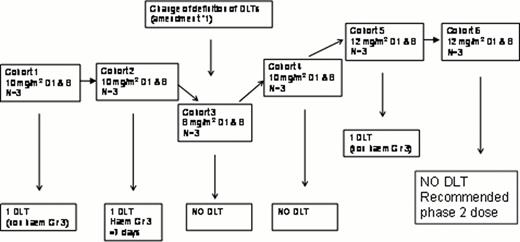
Eighteen patients (11 male, 7 female, aged 31 to 78) have been included and are analyzable for toxicity during the first two cycles. Diagnoses were: PTCL, not otherwise specified (n=10), angioimmunoblastic TCL (n=4), other PTCL (n=4). ECOG performance status was good (0–1) in all but one patient; 17/18 had stage III-IV disease; LDH levels were elevated in 11/18. The age-adjusted IPI score was 0 (n=1), 1 (n=14), 2 (n=3). Significant, albeit tolerable haematological toxicity having been observed in the first two cohorts, the definition of DLT was modified during the course of the study. The study diagram is shown in the figure. Accrual of the phase Ib part of the study is now completed and the phase II part is ongoing with a dose of 12 mg/m2 D1&8. Serious adverse events of interest included: One episode of acute pulmonary edema after course 1 in 1 patient, acute coronary syndrome (n=1), deep venous thrombosis (n=1) and cardiac arrhythmia (n=1). Among 14 evaluable patients, 3 progressed during treatment or shortly after end of treatment; and 11 responded (partial response 3/14, complete response 8/14) for an overall response rate of 78%. The 4 other patients have not yet reached the 8 cycles.
Romidepsin can be combined with CHOP at the price of foreseeable hematological toxicity. Some cardiovascular events have been observed but the relationship with romidepsin is questionable. The dose of 12 mg/m2 on days 1& 8 is currently evaluated in the phase 2 part of the study. Response rates seem promising, but longer follow-up is needed. Updated results will be presented at the meeting.
Ribrag:Servier: Membership on an entity's Board of Directors or advisory committees, Research Funding; astrazeneca: Membership on an entity's Board of Directors or advisory committees; takeda: Membership on an entity's Board of Directors or advisory committees; bayer: Research Funding; sanofi: Research Funding. Coiffier:Celgene: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal