Abstract
Abstract 168
The SPIRIT phase III randomized multicenter open-label prospective trial was designed to compare 4-arms, IM-400 mg versus IM-600mg versus IM-400 mg + cytarabine at a dose of 20 mg/m2/day in cycles of 28 days, versus IM-400 mg + PegIFN at an initial dose of 90 μg per week. The planned molecular analysis after 1 year based on the outcome of 636 pts resulted in a highly significant superiority of superior molecular response (SMR) (0.01 % Bcr-Abl/Abl on IS) of the combination IM 400mg-PegIFN (N Engl J Med, 2010). As of December 31st 2010, date for closing accrual, 787 pts have been included. The current analysis provides update of the trial and additional information on relationship between molecular response and outcomes.
Progression free survival (PFS) was defined by absence of accelerated phase (AP), blast crisis (BC), and death from any reasons, whichever came first. Rates were estimated by the Kaplan-Meier method and compared within groups by the log-rank test. In addition, time to progression (TTP) being defined as AP-BC only, was estimated by cumulative incidence function and compared within groups by the Gray test. Deaths unrelated with progression were then considered as competing events. Pts with available PCR samples at 3 months (N= 665 overall, 197 IM-400mg, 147 IM-600mg, 138 IM-AraC, 183 IM-PegIFN) were classified according to the BCR-ABL cut-off level of 10% IS at 3 and 6 months. Analyses of long term outcomes were then based on the kinetic of molecular response.
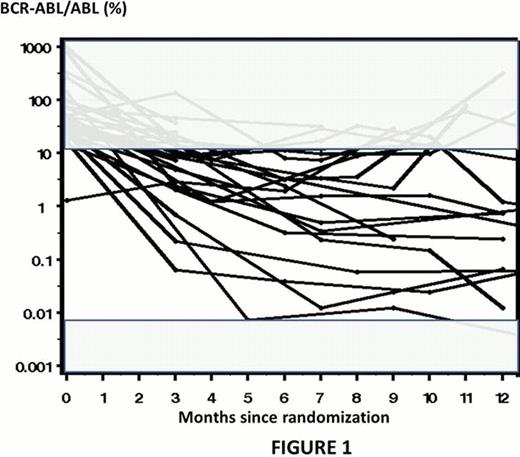
After a median follow-up of 68 months, out of the 787 pts, 59 PFS events (31 AP-BC; 28 deaths in CP) were recorded. Survival without progression at 60 months were for the IM-400mg, IM-600mg, IM-400mg + cytarabine and IM-400mg + PegIFN, 94%, 93%, 90% and 94% respectively (overall, p= 0.24). However we noticed that kinetic of molecular responses of pts who experienced AP-BC was very heterogeneous as showed in Fig 1. The accurate level of bcr-abl/abl transcript and the relevance of the IS conversion factor are questionable when values are above 10% or very low. Thus corresponding plots are shadowed in the Fig1. Then, when pts were stratified according to their molecular response at 3 months, 14 cases of AP-BC and 13 deaths without evidence of progression were recorded in the group of pts with a BCR-ABL ratio <=10% IS (n=522) whereas 14 cases of AP-BC and 9 deaths were recorded in the other group (n=143). Overall, PFS at 3 months was significantly better (p <0.0001) in pts with ratio ≤10% IS. Similarly, TTP was lower (p <0.0001). However, when same analyses were performed according to treatment arm, discrepancies were observed. The potential interest of the 10% BCR-ABL cut-off was still relevant in the IM-400 arm (p <0.0001) and IM-AraC arm (p=0.0199), but no statistical differences were observed in the IM-600 (p=0.6715) and in the IM-PegIFN (p=0.0887) arms respectively. Of interest, these results were confirmed when deaths with no evidence of AP-BC were considered as competing events. The TTP was still significant in the IM-400 arm (p=0.0002) and the IM-AraC arm (0.04). However, no TTP differences were observed in the IM-600 arm (p=0.38); moreover TTP is strictly similar in both molecular response rate groups at 3 months in the IM-PegIFN arm (p=0.96). We also analyzed the cumulative incidence of AP-BC within a period of 6 months according to the kinetic of molecular response (n=568 pts); 3 groups of pts were analyzed: ≤10% within 3 months, ≤10% within 6 months, still above 10% within 6 months. There is a significant advantage (p<0.0001) for early molecular response for all pts included in the trial except for those assigned to the IM-PegIFN arm (p=0.82). Of interest for pts assigned to the IM-PegIFN arm, rate of AP-BC were 3%, 4%, and 0% for the ≤10% within 3 months, ≤10% within 6 months, still above 10% within 6 months groups of pts, respectively.
A 3-months BCR-ABL transcript below the level of 10% IS was associated with a PFS improvement. However, results which were observed with the addition of PegIFN or an increased dose of IM frontline do not confirm the relevance of the 10% BCR-ABL cut-off level as strong surrogate marker for progression to AP-BC. Pts assigned to the IM-PegIFN arm are at very low risk of progression to AP-BC even if their molecular response is delayed.
Mahon:Novartis Pharma: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy, Honoraria; Pfizer: Consultancy. Rea:Bristol Myers-Squibb, Novartis, and Teva: Honoraria. Nicolini:Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; BMS: Honoraria, Research Funding, Speakers Bureau; Ariad: Research Funding, Speakers Bureau; Pfizer: Consultancy, Honoraria. Guerci-Bresler:Novartis, BMS: Speakers Bureau. Guilhot:ARIAD: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal