Abstract
JAK inhibitors have significant palliative benefit in myelofibrosis (MF), mainly in the form of improved constitutional symptoms and reduced splenomegaly. Preliminary data suggests that CYT387, a JAK-1/2 inhibitor, also has the ability to produce anemia responses (ASH Annual Meeting, 2011). In general, the mechanism(s) underlying treatment effects of JAK inhibitors remain unclear but likely represent a drug-specific balance between anti-clonal activity and modulation of immuno cellular-cytokine pathways. We conducted a gene expression profiling (GEP) study using primary cells from MF patients undergoing therapy with CYT387 followed by correlation with clinical data.
Study subjects were enrolled in the Phase-1/2 study of CYT387 treatment in patients with primary (PMF), post-polycythemia vera (PPMF) or post-essential thrombocythemia (PTMF) myelofibrosis. Paired research samples were collected; the time points were pre-study and 12 weeks after commencing study treatment. PBMCs were purified from whole blood by Ficoll separation; RNA was isolated from this cell fraction for GEP analysis. Gene expression profiles were generated using Illumnia Human HT-12 v4 microarray. Pair wise analysis was conducted using the Wilcoxon signed-rank test with a p-value cutoff of 0.05 to generate lists of differentially expressed genes between assigned groups. Pathway analysis was conducted to identify relevant pathways enriched for differentially expressed genes. Comprehensive plasma cytokine profiling was performed using Multiplex Bead-Based Luminex technology (Invitrogen, Carlsbad, CA).
Seventeen patients were studied based on sample availability; 11 (65%) mere male with median age of 66 years (range 53–85). Twelve (71%) were JAK2V617F mutation positive and the DIPSS-plus risk categorization was 10 (59%) high and 7 (41%) intermediate-2. All patients were evaluable for anemia response; 14 (82%) were red cell transfusion dependent at study start. Nine (53%) patients achieved anemia response by IWG-MRT criteria; of these, 8 patients achieved transfusion independence (minimum non-transfused hemoglobin level of 8 g/dL maintained for at least 12 weeks) and 1 had a sustained >2 g/dL increase in hemoglobin level above baseline.
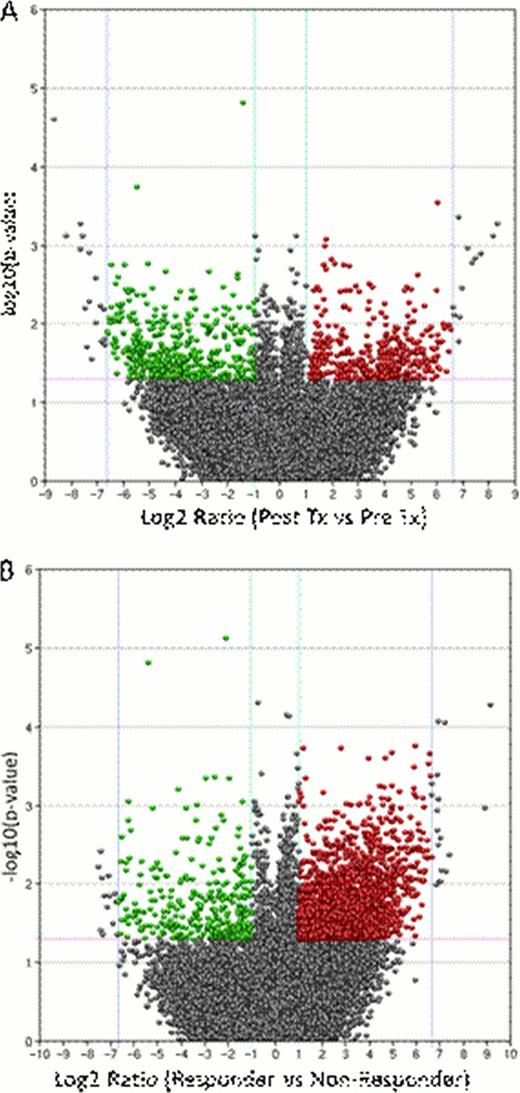
The initial pair wise analysis to identify differential patterns of gene expression compared pre- and post-treatment groups (Figure 1A). This revealed a cluster of significantly (p <0.05) down-regulated genes (minimum 2-fold; median 17-fold) following treatment (displayed in green; upper left quadrant). Pathway enrichment analysis revealed significant associations of these genes with cytokine regulation of immune response, cell proliferation, chemotaxis and cytoskeleton remodeling. We then conducted a pair wise analysis of anemia responders versus non-responders; this revealed a predominance of over expressed gene targets (median 35-fold) in the anemia responder group (Figure 1B) (displayed in red; upper right quadrant). Similar pathway analysis identified enrichment for genes involved in immune system function in this cluster.
The current preliminary analysis suggests that genes relevant to immune response-cytokine pathways are significantly over expressed in patients who achieve anemia response following CYT387 therapy. This further suggests a dominant immune component that underpins ineffective hematopoiesis in responding patients. On the basis of broad treatment-related changes in gene expression we suggest that an important component of CYT387's treatment effect is down regulation of these dysregulated pathways. Ongoing studies include validation of select gene targets which will be tested prospectively in future treatment protocols, as well as correlation of gene expression with circulating cytokine-chemokine levels.
Pardanani:Bristol-Myers Squibb: Clinical trial support, Clinical trial support Other; YM BioSciences: Clinical trial support, Clinical trial support Other; Sanofi-Aventis: Clinical trial support Other. Off Label Use: Data from Phase −1/2 study of CYT387 use in myelofibrosis is mentioned.

This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal