Abstract
Abstract 2266
Betrixaban is a once daily oral Factor Xa inhibitor being investigated in a Phase 3 clinical trial to prevent venous thromboembolism in acute medically ill patients (APEX Study). Mass balance, metabolite profile and interaction with major CYP enzymes were evaluated in this study.
Portola study 06–005 was an open-label, single-dose, mass-balance and metabolic profiling study using 14C-labeled betrixaban in 5 healthy male volunteers. Each subject received a single oral solution containing 40 mg of betrixaban labeled with 100 μCi of 14C. Blood samples were taken serially over a 168-hour interval. Urine samples and fecal samples were collected during the 7–14 day confinement period. Subjects were discharged from the unit when at least one of the following criteria were met: 90% of the radioactivity was recovered in urine and feces, daily excreted radioactivity was 1% or less of administered dose on two consecutive days, or subject reached 336 hours (14 days) post dose. The plasma concentration equivalents of total radioactivity increased rapidly following dosing with a mean peak of 31.69 ng eq/mL occurring at 3.5 hours post-dose. AUC and half-life could not be calculated as radioactivity in plasma could only be detected up to 6 hours post dose. Terminal elimination half life determined in other clinical pharmacology studies was 37 hours. Total radioactivity recovered from urine and feces was approximately 96% (range 92% to 99%), with the majority of 14C recovery in feces (82% to 89% of the dose). The 14C dose recovered in urine, composed of betrixaban and inactive metabolites, ranged from 6% to 13%.
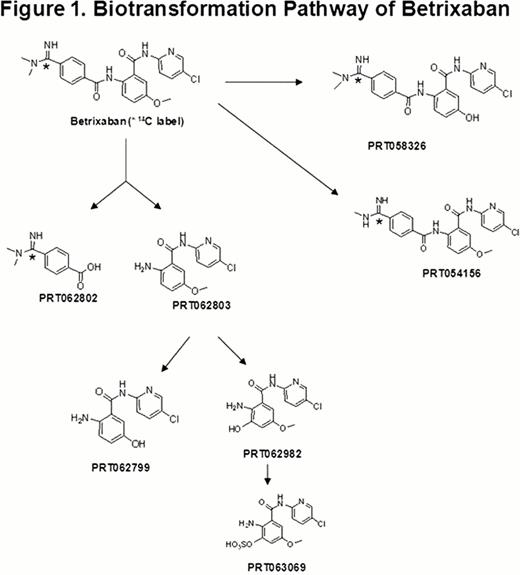
The metabolic profile of betrixaban was determined in plasma, urine and feces. Unchanged betrixaban was the predominant component found in human plasma and excreta, accounting for 85.3% of the dose excreted in urine and feces. The major biotransformation pathway for betrixaban was hydrolysis to form PRT062802 and PRT062803, a non-14C labeled metabolite (Figure 1). PRT062803 can be demethylated to form PRT062799 or hydroxylated to form PRT062982. PRT062982 is further conjugated with sulfate to form PRT063069. Both PRT062802 and PRT063069 were major circulating metabolites in human plasma with AUC of 34% and 24% that of betrixaban, respectively. PRT062802 was the only prominent metabolite detected in human urine and feces. In addition to hydrolysis metabolites, two CYP-mediated metabolites, O-desmethyl betrixaban (PRT058326) and N-desmethyl betrixaban (PRT054156), were observed in plasma at trace levels (AUC of each was <1% that of betrixaban). Trace levels of PRT058326 was also observed in urine and feces. Both PRT062802 and PRT063069 were inactive (IC50 for fXa inhibition >10 μM). PRT058326 and PRT054156 have an IC50 for fXa inhibition of approximately 5 nM compared to betrixaban Ki of 0.117 pM.
Interaction of betrixaban with CYP enzymes was studied in vitro. CYP inhibition potential was evaluated in human liver microsomes with or without 30 minute pre-incubation of betrixaban. Selective probe substrates were used to monitor CYP activities, i.e. phenacetin for 1A2, tolbutamide for 2C9, S-mephenytoin for 2C19, dextromethorphan for 2D6, and testosterone and midazolam for 3A4. Betrixaban had IC50 > 80 μM for CYP1A2, 2C9, 2D6 and 3A4 for both competitive and time-dependent inhibition. IC50 for 2C19 were 43 and 88 μM for competitive and time-dependent inhibition, respectively. The CYP inhibition IC50's are much higher than the betrixaban therapeutic concentration of 50 nM.
CYP induction by betrixaban was also studied using cryopreserved human hepatocytes (n=3). Betrixaban at 1, 10 and 25 μM were incubated in hepatocyte preparation for 48 hours. The activities for CYP1A2, CYP2C9, CYP2C19, and CYP3A4 were determined by measuring the formation of metabolites of the probe substrates similar to those used in the CYP inhibition study. CYP2C19 activities were not quantifiable in all three donors; therefore, induction for this CYP isoform could not be assessed. Betrixaban did not induce the activities of CYP1A2, CYP2C9, and CYP3A4.
These results demonstrated that betrixaban was mainly excreted as the unchanged drug most likely via biliary secretion. Renal excretion and metabolism were minor elimination pathways. Betrixaban is unlikely to have drug-drug interactions with CYP-substrate, inducer, or inhibitor drugs.
Hutchaleelaha:Portola pharmaceuticals: Employment. Ye:Portola Pharmaceuticals: Employment. Song:Portola Pharmaceuticals: Employment. Lorenz:Portola Pharmaceuticals: Employment. Gretler:Portola Pharmaceuticals: Equity Ownership. Lambing:Portola Pharmaceuticals: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal