Abstract
Abstract 2525
Patients with relapsed AML have a very poor outcome. The molecular signature at diagnosis has emerged as a significant marker for prognostic, and possibly therapeutic, values in newly-diagnosed AML. FLT3 mutations are found in about 25–30% of AML pts. In the absence of FDA-approved agents for FLT3, the prognostic value of FLT3 mutation on clinical outcome for relapsed AML (rAML) is not well documented.
To study clinical implications of FLT3 status on rAML.
All adult pts with AML diagnosed and treated at Mayo Clinic between 2003–2011were studied. Retrospective data collection included pts' demographics, laboratory tests, bone marrow biopsies, FLT3 mutation (internal tandem duplication (ITD) and tyrosine kinase domain (TKD) mutation), and survival information. IRB approval was obtained in accordance with Helsinki declaration. Chi-Square and T-test were used for comparatives between groups and Kaplan-Meier test was applied for survival estimates using JMP 9.0 software.
A total of 192 pts with newly-diagnosed with AML were identified. Sixty percent (115) were males, 88% (168) were Caucasians, 26% (49) had antecedent hematological disease (AHD), and 13% (25) had previous exposure to chemo- or radiotherapy. Forty-seven percent had a normal chromosomal analysis, and 18% (35) had FLT3 mutation (28 ITD, 7 TKD). In FLT3-mutated pts, median WBC was 70 x109/L (p=0.0016), hemoglobin 9.5 g /dL, and platelets 48 x109/L, compared to 8.8 x109/L, 9.1 g/dL, 50 x109/L in the rest of pts, respectively. Peripheral blood blasts were elevated in FLT3-mutated group compared to rest of pts (p 0.0059).
A total of 128 pts achieved complete remission (CR), with 79 (62%) subsequently relapsed. Of the relapsed patients, 93% (74) were Caucasians, 54% (43) males, 22% (17) had AHD, and 8% (6) had prior chemo- or radiotherapy. Cytogenetics were diploid in 49% (39) and complex in 15% (12). Hematopoietic stem cell transplantation was performed in 30% (24) of pts. Eleven of 79 (14%) pts were FLT3-mutated at relapse (Group 1); with 1 pt having TKD-mutation. Forty-eight percent (38) of pts had unmutated-FLT3 (Group 2), and 38% (30) pts were FLT3-unknown (Group 3).
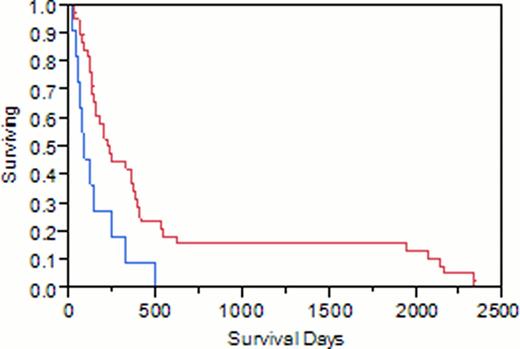
In group 1, median WBC was 9.9 x109/L, hemoglobin 10.55 g /dL, platelets 55 x109/L, compared to 3.2 x109/L (p=0.0034), 10.55 g/dL, and 58 x109/L in group 2, respectively. Peripheral blasts were significantly elevated in group 1 compared to group 2 (38% vs 4%, p <0.0001). Surprisingly, 3/11 pts (27%) with FLT3 mutation at relapse were unmutated at diagnosis. CR was achieved in 2/11 (18%) pts in group 1 compared to 15/38 (39%) in group 2 (p=0.19). Median overall survival in rAML was significantly lower in group 1 compared to group 2 (90 days vs 229 days, p 0.0076). This survival difference was statistically more significant in pts with diploid cytogenetics at relapse compared to the rest (p 0.0009 vs 0.48, respectively). Within 1.5 years, all pts in group 1 died, compared to 21% survived in group 2.
FLT3-mutated AML pts had a significantly higher peripheral WBC and blasts at both diagnosis and relapse. Importantly, FLT3 at relapse was found to be mutated in one quarter of pts who were wild-type FLT3 at diagnosis; raising the importance of assessing FLT3 gene on all relapses. FLT3 mutation significantly predicted worse median overall survival in rAML raising the need for novel agents, such as FLT3 inhibitors, in this population.
Flt3 mutated blue, flt3 wild type red.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal