Abstract
Abstract 3555
Although treatment-related acute myeloid leukemia (tr-AML) has been identified as an independent marker of poor prognosis, it is important to determine how characteristics of the patient, primary malignancy, or treatment regimen affect the development and outcome of tr-AML. Taxane therapy has become essential in the management of breast cancer in light of its favorable effect on survival in short term studies. However, clinical trials fail to capture acute leukemias related to recently introduced agents since development of tr-AML occurs years after chemotherapy.
To examine the effect of taxane therapy on clinical outcome of patients who were treated for breast cancer and subsequently developed tr-AML.
This retrospective chart review considers patients diagnosed with AML at Mayo Clinic between 1990 and 2011. Patients were included if they had previously received chemotherapy for breast cancer, or if they had never received any chemotherapy. Demographic data, previous diagnosis and treatment regimen, CBC at diagnosis, pathology and cytogenetic information, therapy used, and response were collected for each patient. IRB approval was obtained. Comparison between two groups was done using t-test; survival estimates using Kaplan-Meier curves were constructed with software JMP 9.
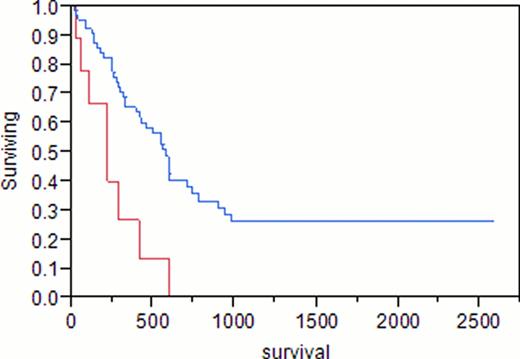
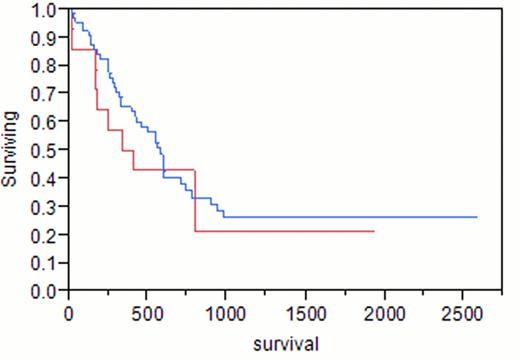
A total of 88 adult females with AML were identified. Median age at AML diagnosis was 58 years (range 19–86). Median hemoglobin was 8.9 g/dL (4–14.5), white blood cell count (WBC) 9.7 x109/L (0.6–237), platelets 67 x109/L (1–361), peripheral blood (PB) blasts 13%, bone marrow blasts (BM) 44%, and BM cellularity 90%. Of the 88 patients, 23 (26%) had previously received treatment for breast cancer (tr-AML), the remainder had de novo AML. Taxanes were included in the initial treatment for breast cancer in 14 (61%, median age 57, group 1), compared to 9 (39%) patients with no history of taxane exposure (median age 58, group 2). Median hemoglobin was 9.7 g/dL, WBC 4.5×109/L, platelets 72×109/L, PB blasts 2%, BM blasts 40% in group 1, compared to 8.3, 7.1, 50, 28%, 47%, respectively, in group 2. Cytogenetic analysis of group 1 showed diploidy in 8%, complex karyotypes in 8%, and chromosome 11 abnormalities in 38%, compared to 50%, 0%, 33%, respectively, in group 2. In group 1, 14% had good risk cytogenetics, 57% had intermediate risk, and 29% had poor risk, compared to 17%, 83%, and 0%, respectively, in group 2. Cytogenetic analysis of de novo AML showed diploidy in 71%, complex karyotypes in 14%, and chromosome 11 abnormalities in 0%. Complete remission (CR) was achieved in 79% of group 1 and 83% of group 2 (p 0.8). Differences in survival of group 1 (375 days) and group 2 (222 days) did not reach statistical significance (p 0.12). Median survival was 584 days for patients with de novo AML, 375 days in group 1 (p 0.47), and 222 days in group 2 (p<0.001).
In agreement with previous reports, we found that tr-AML is a poor prognostic factor overall (p 0.04). However, patients with a history of breast cancer who had been treated with taxanes as a part of their initial chemotherapy regimen demonstrated similar survival as patients with de novo AML (p 0.47). Tr-AML patients only did significantly worse than patients with de novo AML if they had never received taxanes as part of their breast cancer therapy (p<0.001).
Blue = de novo AML; Red = tr-AML, no taxane exposure
p = 0.0009
Blue = de novo AML; Red = tr-AML with taxane exposure
p = 0.479
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal