Abstract
Abstract 3652
Patients with classical Hodgkin lymphoma (cHL) who fail stem cell transplantation have very poor prognosis and new drugs with encouraging activity and low toxicity profile are required. Recently, Bendamustine has demonstrated efficacy as single agent in several hematological malignancies and it represents a suitable alternative for cHL patients.
Patients with relapsed/refractory cHL after high-dose chemotherapy and autologous or allogeneic transplant, were treated with Bendamustine as single-agent in 16 FIL Centers. Endpoints included overall response rate (ORR), adverse events and survival. All patients provided written informed consent at the time of treatment. This observational study was approved by Ethical Committees at all sites.
From October 2007 to May 2012, 73 patients were included in a single-agent salvage program with Bendamustine. Of them, 69 were eligible for the analysis. Median age was 34 yrs (range, 21 – 63). Bendamustine was administered to cHL patients refractory to, or relapsing after autologous (n = 46) or autologous and allogeneic (n = 23) transplants. Forty-eight were refractory to previous treatment, whereas 21 were relapsed patients, including 11 early relapse (<12 months) and 10 late relapse (>12months). Three dosages of Bendamustine were employed: 90mg/smq, 100mg/smq and 120mg/smq for 2 consecutive days every 28 days. The median number of administered cycles was 4 (range, 1 – 12).
ORR was 58%, including 25% complete remission (CR) and 33% partial response (PR). Thirty-eight percent experienced progression and 4% had stable disease. Median time to response was 3.4 months (range; 1.4 – 10). Median duration of response was 5.1 months (range; 0.1 – 23).
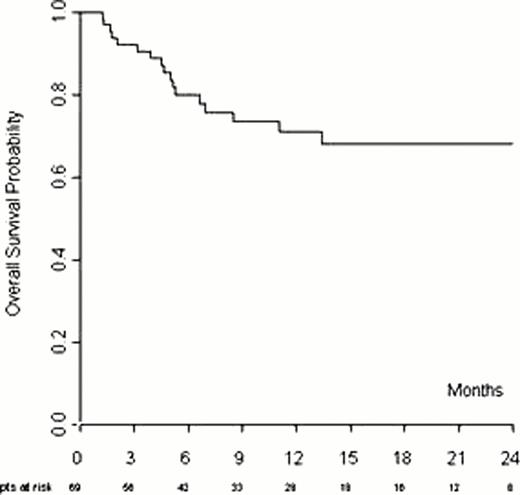
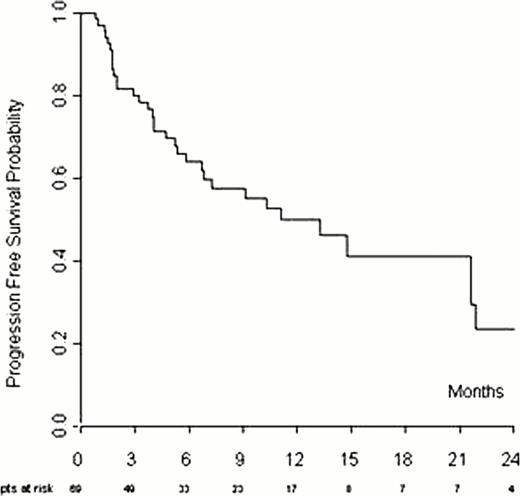
In multivariate analysis factors affecting the response are: refractory disease and more than 3 previous chemotherapy lines. With a median follow-up of 13 months (range; 1 – 32), median progression free survivals (PFS) was 10 months while median overall survival (OS) was not reached. The 1 and 2-year progression-free survival (PFS) were 50% and 24%, respectively (Figure 1). The 1 and 2-year OS were 71% and 68%, respectively (Figure 2). Bulky disease, ECOG>0 and lack of CR to Bendamustine have a negative impact on outcome in multivariate analysis. Dose-reduction was required in 22% of the patients with no impact on response (p 0.613). Twenty patients received GCS-F, mainly in the 120 mg/sqm cohort. Drug-related toxicity of any WHO grade was documented in 33/69(48%) of patients. Severe hematologic toxicity was observed in 20/69 pts (29%); grade 3–4 neutropenia, thrombocytopenia and anemia were observed in 11 (16%), 11(16%) and 4(6%)pts, respectively. Of note only one patient required drug discontinuation due to severe thrombocytopenia. Infection was the most common nonhematologic toxicity. Severe infections, mainly sepsis, occurred in 5pts but no one required drug discontinuation.
In our experience, Bendamustine is a useful single-agent with manageable toxicity for cHL patients with relapsed/refractory disease after autologous or allogeneic transplant. These results support prospective strategies with Bendamustine alone or in combination as pretransplant induction regimen.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal