Abstract
Abstract 3957
Currently used clinical prognostic markers for patients with multiple myeloma (MM) such as the international staging system (ISS) and cytogenetics are inadequate predictors of response and survivorship after hematopoietic stem cell transplant (HSCT). Recently published large studies of HSCT as consolidation after primary therapy have demonstrated 3-year progression free survival (PFS) and overall survival (OS) rates of 45% and 78% respectively after tandem HSCT (BMT CTN 0102; Krishnan, Lancet Onc, 2011) and median time to progression of 46 and 27 after single autologous transplant, with and without lenalidomidemaintenance (CALGB 10104; McCarthy, NEJM, 2012). These studies (which include patients in the current cohort) have established expected PFS and OS benchmarks that allow identification of higher risk subsets.
The aim of this study is to describe and further sub- stratify patients with high-risk myeloma (HRMM), with the goal to identify ‘higher’ risk groups that may benefit from alternative treatment strategies.
A retrospective cohort study of HRMM patients who received an HSCT at OHSU between 01/01/2001 and 12/31/2011 was performed. We defined HRMM by the following: FISH and cytogenetic findings of del17p, t(4:14), t(14;16), t(14;20), chromosome (ch) 1 abnormalities, del 13q by cytogenetics; the presence of multiple extra-medullary plasmacytomas; plasmablastic morphology; higher ISS categories (II and III); Salmon- Durie(S-D) stages 2 and 3; recurrence or less than a partial remission (PR) to 2 consecutive lines of therapy prior to HSCT.
Outcome measures included PFS and OS. Descriptive statistical analysis was conducted for all primary and secondary endpoints, patients' individual and clinical characteristics, and gene profiles. Kaplan-Meier method was used to estimate the OS and PFS function. Log-rank test was used to assess whether the survival function differs across the groups. Factors that are significantly associated with the primary and secondary endpoints were identified using univariateanalysis. Multivariate analysis is ongoing.
Patient and HSCT characteristics are found in table 1.
n = 252
| Characteristic . | No . | % . |
|---|---|---|
| Age at transplant, years | ||
| Median | 59 | |
| Range | 30–79 | |
| Male Sex | 162 | 64 |
| ISS stage | ||
| II | 89 | 45 |
| III | 47 | 24 |
| Salmon-Duriestage | ||
| 2 | 57 | 23 |
| 3 | 145 | 58 |
| Genetic abnormalities | ||
| del (13q) cytogenetics | 30 | 11 |
| t(4;14) | 21 | 8 |
| del(17p) | 18 | 7 |
| t(14;16) | 4 | 1 |
| t(14;20) | 1 | 0.3 |
| ch 1 abnormality* | 21 | 9 |
| Plasmablastic morphology | 6 | 2 |
| Multiple extramedullary plasmacytomas | 18 | 7 |
| Prior therapies | ||
| Median | 2 | |
| Range | 1–9 | |
| <PR to 2 conventional lines of therapy prior to HSCT | 24 | 9 |
| HSCT total | 273 | |
| Single Autologous | 242 | |
| Tandem | 7 | |
| Allogeneic | 24 | |
| Sibling | 14 | |
| MUD | 9 | |
| Syngeneic | 1 | |
| Indication for HSCT | ||
| Induction | 207 | 76 |
| Salvage | 66 | 24 |
| Post HSCT maintenance | 51 | 19 |
| Lenalidomide | 19 | |
| Bortezomib | 2 | |
| Steroids | 12 | |
| Thalidomide | 18 |
| Characteristic . | No . | % . |
|---|---|---|
| Age at transplant, years | ||
| Median | 59 | |
| Range | 30–79 | |
| Male Sex | 162 | 64 |
| ISS stage | ||
| II | 89 | 45 |
| III | 47 | 24 |
| Salmon-Duriestage | ||
| 2 | 57 | 23 |
| 3 | 145 | 58 |
| Genetic abnormalities | ||
| del (13q) cytogenetics | 30 | 11 |
| t(4;14) | 21 | 8 |
| del(17p) | 18 | 7 |
| t(14;16) | 4 | 1 |
| t(14;20) | 1 | 0.3 |
| ch 1 abnormality* | 21 | 9 |
| Plasmablastic morphology | 6 | 2 |
| Multiple extramedullary plasmacytomas | 18 | 7 |
| Prior therapies | ||
| Median | 2 | |
| Range | 1–9 | |
| <PR to 2 conventional lines of therapy prior to HSCT | 24 | 9 |
| HSCT total | 273 | |
| Single Autologous | 242 | |
| Tandem | 7 | |
| Allogeneic | 24 | |
| Sibling | 14 | |
| MUD | 9 | |
| Syngeneic | 1 | |
| Indication for HSCT | ||
| Induction | 207 | 76 |
| Salvage | 66 | 24 |
| Post HSCT maintenance | 51 | 19 |
| Lenalidomide | 19 | |
| Bortezomib | 2 | |
| Steroids | 12 | |
| Thalidomide | 18 |
Ch 1 abnormalities were heterogenous and included 8 deletions (1q x1, 1p x7); 4 additions (1p x2, 1q x2); 5 translocations (t1;4, t1;8, t1;19, t1;10 x2) and 4 with multiple abnormalities.
With a median follow up of 40 months, relapse occurred in 127 patients, of which 77 (60%) occurred within 18 months post HSCT. Median PFS and OS are 22.3 (95% CI: 19.7 – 29.3) and 56.67 (95% CI: 40.1–69.9) months respectively. The 2-year PFS and OS rates were 47%, and 72% respectively.
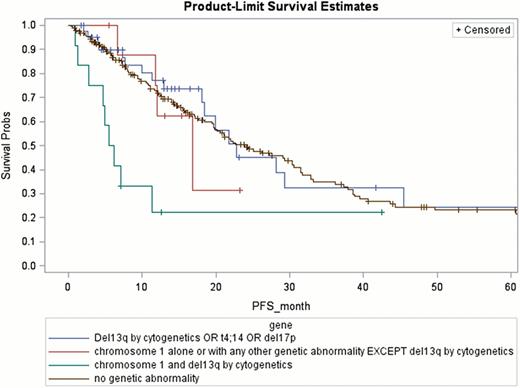
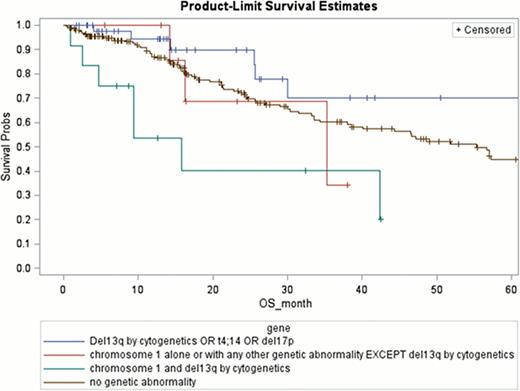
Univariate data analysis revealed the following factors that are highly associated with reduced PFS: del 17p (2- year PFS 26.2%; p= 0.09); ch 1 abnormalities (27.4%; p=0.0026); recurrence or < PR after 2 consecutive lines of chemotherapy prior to HSCT (27.5%; p= 0.07). For patients with chromosome 1 abnormalities, the presence of del 13q by cytogenetics further decreased the PFS (22%; p= 0.04;)(Figure 1). Factors associated with highly with a reduced OS are: ch 1 abnormalities (2-year OS 52.5%; p=0.0042); both chromosome 1 and del13q (46.2%; p=0.0016) and having multiple extra-medullary plasmacytomas (55.2%; p= 0.026 (Figure 2).
Within the broad group of HRMM, certain groups have significantly inferior outcomes post HSCT, the worst being those with recurrence or < PR to 2 consecutive lines of chemotherapy prior to HSCT, those with any ch 1 abnormality and particularly those with additional del 13q by cytogenetics. Investigation of novel therapeutic and more aggressive strategies is warranted in these groups.
Scott:Genzyme: Research Funding; Millenium: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal