Abstract
Abstract  4098
4098
Relapse after allogeneic hematopoietic cell transplantation (HCT) is usually incurable for acute myeloid leukemic (AML) patients. Residual disease (MRD) monitoring pre- and post-HCT may improve relapse prediction, allowing the targeted implementation of post-HCT interventions to at risk patients when disease burden is sufficiently low for these to be effective. Previous studies have shown that MRD detection by multiparameter flow cytometry (MRD MFC) at either pre- (Leung et al 2012, Walter et al 2011) or post- transplant (Yan et al 2012) timepoints is prognostic for myeloablative HCT outcome in AML. Quantification of hematopoietic populations enriched for leukemic stem cells such as CD34+CD38low or lymphoid-primed multi-potential progenitor-like (LMPP-like) (Lin-CD34+CD38lowCD90-CD45RA+) (Goardon et al 2011) may improve the specificity of MFC MRD assays.
In this study we retrospectively evaluated the predictive value of MRD MFC at both pre- and post- HCT timepoints in a cohort of unselected AML/high risk myelodysplasia (MDS) patients (n=44) who underwent reduced intensity conditioning (RI n= 32, median age 59, range 34–70) or myeloablative (MA n=12, median age 28, range 19–47) HCT between June 2010 and November 2011 (Table 1). MFC MRD was assessed both by detection of standard leukemic- aberrant-immunophenotypes (LAIPs) (identified at presentation and/or relapse) and quantification of CD34+CD38low and LMPP-like progenitors (LSC-enriched progenitors, LSC-EP).
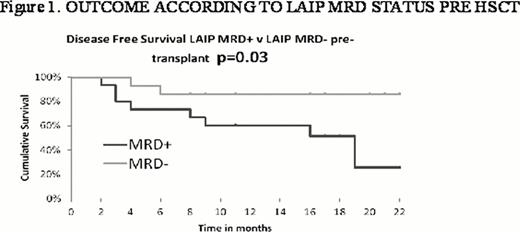
Pre HCT, 37 patients (MA = 11, RI = 26) were assessable for MFC MRD. 15 (41%) were MRD positive (LAIP MRD+) and 47% (7/15) (MA = 37.5%, 3/8; RI = 57%, 4/7) of these relapsed post HCT compared to 8% (MA = 0%, RI = 9%) of MRD negative patients (LAIP-MRD-), (Fig 1 p 0.03).
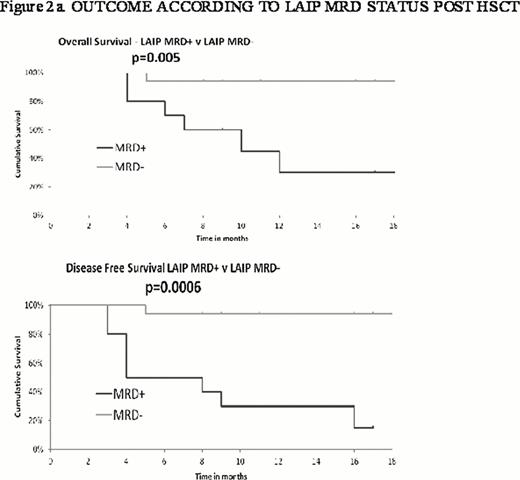
Post HCT, 34 patients (MA = 9, RI =25) were assessable for MFC MRD. 10 (37%) had detectable LAIP MRD positivity between 2 and 9 months post HCT. 80% of these relapsed (MA = 60%; RI = 100%) with a median disease free survival (DFS) post HCT of 5 months (MA = 4 months; RI = 5 months); there were no relapses in the 17 patients who remained LAIP MRD- at a median follow-up of 20 months (range 9–26). (p=0.0006, Figure 2a). Presence of MRD post-transplant was associated with significantly poorer overall survival (p=0.005).
Although 1 patient with high MRD (in CR, LAIP >1%, LSC-EP +) pre HCT relapsed <3 months post HCT, in 8 of 9 other relapses MRD positivity detected at ≥ 2 months post HCT preceded clinical relapse by >1 month (median time to relapse from MRD detection of 1.5 months, range 1–6; OS, median 4 months, range 1 - not reached).
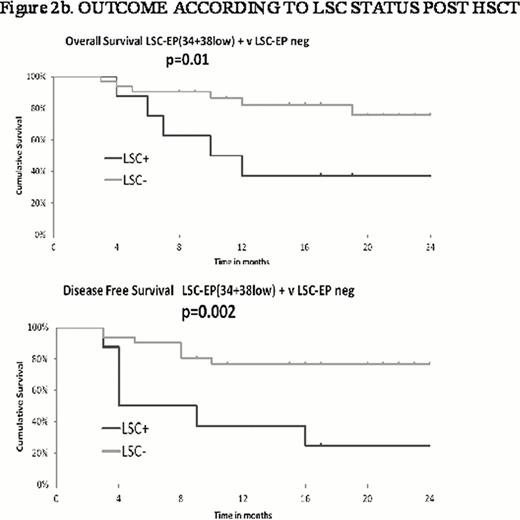
CD34+CD38low progenitors (34+38low) were < 0.03% of bone marrow nucleated cells in the majority of patients. Detectable 34+38low were mainly CD45RA+ so in most cases correlated with LMPP-like quantitation. Pre HCT, 34+38low were detectable in 40% of patients who went on to relapse and in only 9% of those who have not yet relapsed. Post HCT, 34+38lowpositivity preceded frank relapse by ≥1 month in 60% of patients who relapsed. Only 6% of patients who have not yet relapsed had detectable 34+38low. LSC-LEP positivity appears prognostic for DFS and OS (Figure 2b) but for a lower frequency of relapses compared to LAIP MRD positivity (60% v 80%).
These data suggest that post HCT MFC detection of LAIP MRD is predictive of relapse in RI as well as MA HCT. LSC-LEP quantitation may be prognostic in a subset of patients. Pre HCT MRD might be more predictive of relapse in RI than MA HCT. However, post HCT MRD positivity precedes most clinical relapses by a time window which may be sufficient for interventions such as azacytidine or donor lymphocyte infusion (DLI) when disease burden is still low. These results provide a basis for the use of MFC residual disease detection pre and post HCT to inform treatment decisions in reduced intensity as well as myeloablative HCT.
Patient Demographics
| . | Total . | LAIP MRD pre HCT . | LAIP MRD post HCT . | ||
|---|---|---|---|---|---|
| +ve . | -ve . | +ve . | -ve . | ||
| No. of patients | 44 | 15 | 14 | 10 | 17 |
| Median Age (range) | 55 (19–70) | 41(19–66-) | 60 (26–66) | 44(19–66) | 61(20–66) |
| Sex | |||||
| Female | 18 | 5 | 5 | 3 | 7 |
| Male | 26 | 10 | 9 | 7 | 10 |
| Type of HCT | |||||
| Reduced intensity | 32 | 8 | 12 | 5 | 14 |
| Myeloablative | 12 | 7 | 2 | 5 | 3 |
| Remission status pre HCT | |||||
| CR1 | 30 | 9 | 12 | 10 | 13 |
| CR2/CR3 | 13 | 5 | 2 | 0 | 3 |
| Refractory | 1 | 1 | 0 | 0 | 1 |
| Cytogenetics | |||||
| Favourable | 1 | 1 | 0 | 0 | 1 |
| Intermediate | 35 | 7 | 13 | 7 | 13 |
| Adverse | 8 | 7 | 1 | 3 | 3 |
| . | Total . | LAIP MRD pre HCT . | LAIP MRD post HCT . | ||
|---|---|---|---|---|---|
| +ve . | -ve . | +ve . | -ve . | ||
| No. of patients | 44 | 15 | 14 | 10 | 17 |
| Median Age (range) | 55 (19–70) | 41(19–66-) | 60 (26–66) | 44(19–66) | 61(20–66) |
| Sex | |||||
| Female | 18 | 5 | 5 | 3 | 7 |
| Male | 26 | 10 | 9 | 7 | 10 |
| Type of HCT | |||||
| Reduced intensity | 32 | 8 | 12 | 5 | 14 |
| Myeloablative | 12 | 7 | 2 | 5 | 3 |
| Remission status pre HCT | |||||
| CR1 | 30 | 9 | 12 | 10 | 13 |
| CR2/CR3 | 13 | 5 | 2 | 0 | 3 |
| Refractory | 1 | 1 | 0 | 0 | 1 |
| Cytogenetics | |||||
| Favourable | 1 | 1 | 0 | 0 | 1 |
| Intermediate | 35 | 7 | 13 | 7 | 13 |
| Adverse | 8 | 7 | 1 | 3 | 3 |
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal