Abstract
Abstract 4549
Patients with high risk AML have poor outcomes. However, the only approach with curative potential remains allogeneic HSCT. With the aim to improve the effect of allogeneic HSCT by sequential use of chemotherapy, RIC regimen and pDLI, we are currently conducting a prospective pilot study for high risk AML. High risk AML was defined by unfavorable cytogenetics, adverse molecular abnormality, secondary AML, and AML requiring 2 induction courses to obtain CR1.
After identification of a HLA 10/10 donor, patients are received the sequential regimen consisted of fludarabine (30mg/m2/d), Ara-c (2g/m2/d) and amsacrine (100mg/m2/d) (FLAMSA) chemotherapy for 4 days. After 3 days of rest, RIC regimen consisted of 4 Gy TBI, cyclophosphamide for 2 days (40 mg/kg in case of matched related donors, and 60 mg/kg for unrelated or mismatched donors), and ATG (5mg/kg total dose) (German regimen) or Busulfan 3.2mg/kg/d during 4 days followed by ATG (5mg/kg total dose) (French regimen). The modified regimen has been established after our results in refractory AML patients (ASH 2011, poster 1957). Prophylactic donor lymphocyte transfusion was given from day +120 in patients who were not receiving immunosuppression and were free of GvHD.
Our objective is to include 20 patients and to compare with a control cohort of patients with the same high risk AML treated according to the conventional strategy during the same period.
Between August 2010 and November 2011, we have included 12 consecutive patients in first complete response who underwent an allogeneic HSCT after sequential FLAMSA-RIC regimen with a median follow-up of 12 months (range [7–22]). Nine patients were < 55 years old (median age: 54 [28–64]), 7 patients had an unrelated donor and 5 patients had a related donor. The stem cell source was PBSC for 11 patients and two cord blood unit for 1 patient. Before FLAMSA-RIC regimen, 3 patients had received two induction courses to obtain CR1. All patients had adverse cytogenetics or molecular abnormalities and 1 patient had a secondary AML.
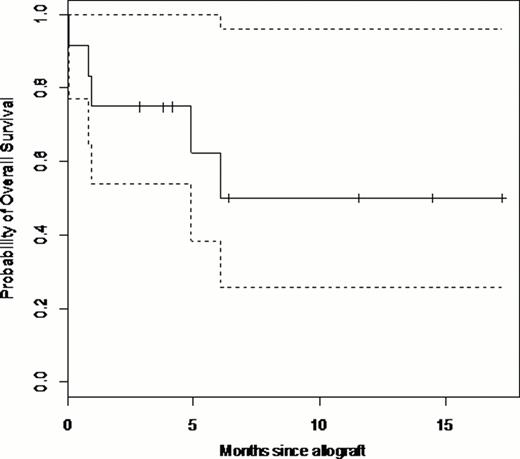
At the last follow-up, 6 patients (50%) are alive in CR. (Figure 1.) Four patients (33.3%) died in remission. The cause of death was infection for 2 patients, aGvHD for 1 patient and graft failure for 1 patient. Only one patient died from relapse 6 months after transplantation.
Five patients (41.6%) experienced aGvHD and 2 patients (16.6%) had an extensive cGvHD including the patient who has been transplanted with 2 cord blood unit. Six patients (75%) in a group of 8 patients aged > 45 years experienced complications (infection (n=3) and GvHD (n=3)). One patient (25%) from a group of 4 patients aged < 45 years had infectious complication after transplantation. Prophylactic donor lymphocyte transfusion was given in 6 patients, the causes of no administration were GvHD for 2 patients, cord blood unit as stem cell source for 1 patient and 3 patients were dead before 120 days after transplantation. From the 6 patients who had received pDLI, 5 patients are alive in CR and 1 patient died from GvHD.
The FLAMSA-RIC regimen before allogeneic HSCT is a new approach for high risk AML. Between 2012 January and 2012 July, 8 additionnal patients have been included and the results for the whole study will be communicated later. Our primary results are promising especially for young patients (< 45 years) who seem to better profit from this sequential FLAMSA-RIC regimen.
Nicolini:Novartis, Bristol Myers-Squibb, Pfizer, ARIAD, and Teva: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal