Abstract
Abstract 4548
High-dose chemotherapy followed by autologous stem cell transplantation (ASCT) is currently standard treatment for younger patients with multiple myeloma (MM). In the face of almost inevitable disease relapse, there is growing evidence for second ASCT as salvage therapy in certain patient groups. However, few published data exist regarding efficacy and safety of third ASCT in relapsed disease.
We retrospectively analysed the results of eight patients treated at a single UK institution who each received three separate autologous stem cell transplants for relapsed MM between May 1997 and April 2012. There were four men and four women. Median age at diagnosis was 48 years (range, 25–64 years). Paraprotein isotype was IgA in two patients and IgG in the remaining six patients.
At the time of 1st transplant, seven patients were in partial response (PR) and one in complete response (CR). Conditioning melphalan dose was 200mg/m2 in all but two patients who received 140mg/m2. Three patients entered CR following 1st transplant and four patients showed PR. Median time to disease progression was 31 months (range, 11.8–52.9 months). Prior to 2nd transplant, five patients achieved very good partial response (VGPR) and three PR with induction chemotherapy. Melphalan dose was 200mg/m2 in five patients and 140mg/m2 in the remaining three. Median time to disease progression was 22.3 months (range, 10.1– 39.6 months). At the time of 3rd transplant, two patients had achieved VGPR following induction chemotherapy, one showed PR, two stable disease (SD) and three evidence of disease progression. For the 3rd transplant, melphalan dose was reduced in most cases.
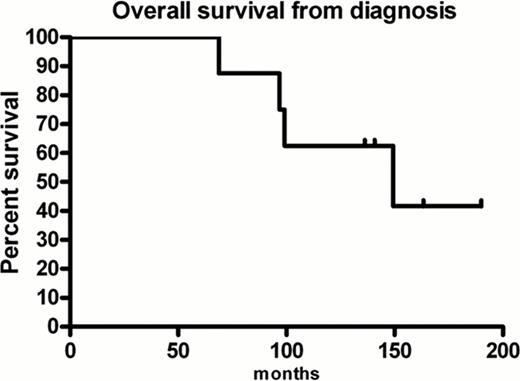
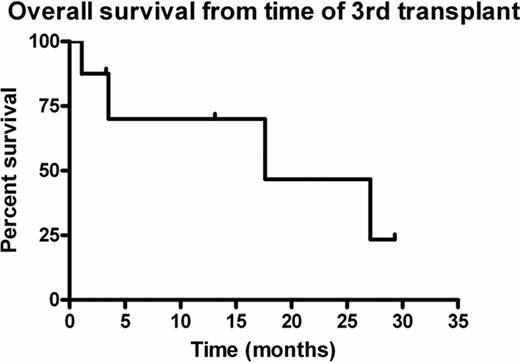
Median follow up post 3rd transplant was 8.3 months (range 1.1–29.3 months). One patient died of overwhelming sepsis within one month of transplantation (treatment related mortality). At the time of analysis, five patients had relapsed following 3rd ASCT, with median time to disease progression of 10.4 months (range, 2.7–23.7 months). Three of these patients died at 3.5, 17.6 and 27.1 months post 3rd transplant. The remaining two patients are alive with no evidence of disease relapse (progression free survival (PFS) time of 3.3 and 1.3 months). Overall survival (OS) for the group from diagnosis is 62% at 10 years with a median OS from diagnosis of 149 months (range 68.5 – 189.2 months) (Figure 1 ). Median OS for the group from 3rd transplant is 17.6 months (range, 1.1–29.3 months) (Figure 2 ). Median PFS is 10.4 months (range 1.1–23.7 months).
These results demonstrate that third ASCT is a possible treatment strategy for patients with relapsed MM and may prolong patient survival.
Overall survival from diagnosis for patients receiving 3rd autologous stem cell transplantation for relapsed multiple myeloma
Overall survival from diagnosis for patients receiving 3rd autologous stem cell transplantation for relapsed multiple myeloma
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal