Abstract
Abstract 733
In 2005, the HCT-CI was introduced by a single institution as a weighted scoring system to predict mortality risk following allogeneic HCT. Since then, not all investigators were able to validate the HCT-CI after testing in their respective institutions. In 2007, a new prospective multi-institutional observational study was initiated at the CIBMTR to collect comorbidities from all transplant centers by their respective evaluators and to validate the predictive power of the HCT-CI in a large sample of patients (pts). The HCT-CI was adapted into the Pre-Transplant Essential Data (pre-TED) collection form #2400. Data managers from all institutions attended an education session on comorbidity coding per the HCT-CI at the 2007 Tandem BMT Meeting in Keystone, Colarodo. This session was then made public to all data managers at the CIBMTR website. <>The study accrued 8115 consecutive pts treated with allogeneic HCT from 12/2007 to 12/2009 from related (47%) or unrelated (53%) donors. Median age was 52 [range 1–78) years. Conditioning regimens were high-dose (67%) or either reduced-intensity (RIC) or nonmyeloablative (NST) regimens (34%). Diagnoses were acute (54%) or chronic (12%) leukemia, myelodysplastic syndromes (16%), lymphomas (16%), and others (2%). GVHD prophylaxis regimens were cyclosporine-based (22%), tacrolimus-based (68%), or others (10%). Stem cell source was marrow (17%) or peripheral blood mononuclear cells (83%). Karnofsky performance status scores were <90% (33%), ≥ 90% (62%), or missing (5%). HCT-CI scores were 0 (47%), 1 (15%), 2 (11%), 3 (12%), 4 (7%), 5 (3%), ≥6 (4%), or missing (1%). About 11% of pts with score 0 had other comorbidities listed.
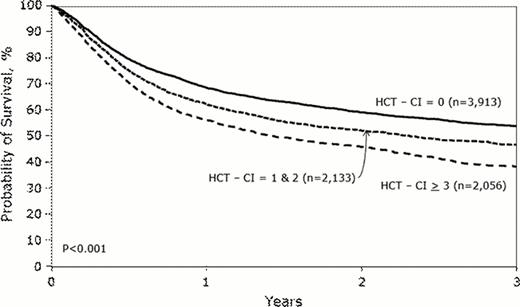
Overall, pts experienced cumulative incidence of transplant-related mortality (TRM) of 28% and a survival rate of 48% at 3-years. Pts with HCT-CI scores of 0 vs. 1–2 vs. ≥3 had 3-year TRM incidences of 24%, 28%, and 35% (p <0.001) and 3-year overall survival (OS) rates of 54%, 47%, and 38%, respectively (p <0.001, Figure). Proportional hazards models were used to estimate the hazard ratio (HR) for TRM and OS associated with HCT-CI scores. The models were adjusted for all previously mentioned covariates in addition to disease status, CMV serology status, gender, and race. Increasing HCT-CI scores (1–2 and ≥3 vs. 0) were associated with increases in the HR [95% confidence interval (CI)] for TRM [1.12 (1.00–1.26) and 1.47 (1.31–1.65), respectively, p<0.0001] and OS [1.12 (1.03–1.22) and 1.36 (1.25–1.48), respectively, p<0.0001] in the overall pt population. No statistically significant difference could be detected between pts with score 0 + other comorbidities vs. score 0 for TRM (HR 0.93, p= 0.385) or OS (HR 0.96, p= 0.474). When the HCT-CI was modeled as scores of 0, 1, 2, 3, 4, and ≥5 the HR for TRM were 1.00 vs. 1.12 vs. 1.13 vs. 1.31 vs. 1.52 vs. 1.77, respectively (, p<0.0001) and for OS were 1.00 vs. 1.13 vs. 1.12 vs. 1.22 vs. 1.39 vs. 1.62 (p<0.0001). Likewise, the HCT-CI could discriminate outcomes well among pts given high-dose or RIC/NST regimens and those diagnosed with lymphoid or myeloid diseases (Table 1).
The inter-rater reliability (IRR) rate among data managers versus their respective investigators was assessed in 3 institutions. Weighted kappa statistics were 0.54, 0.81, and 0.47 respectively, indicating fair-moderate agreement rate among evaluators.
The HCT-CI is a valid tool to discriminate relative risks for TRM and OS after HCT across different institutions, different conditioning intensities, and different diagnoses. The HCT-CI should be used as a standard-of-care health measure in counseling pts for HCT, in clinical trial design, and in adjusting statistical analyses for HCT outcomes. Future efforts will focus on improving the IRR of the HCT-CI.
Multivariate analyses
| . | . | TRM . | OS . | ||
|---|---|---|---|---|---|
| . | HCT-CI scores . | HR . | p-value . | HR . | p-value . |
| High-dose regimens | 0 | 1.00 | <0.0001 | 1.00 | <0.0001 |
| 1 | 1.19 | 1.14 | |||
| 2 | 1.12 | 1.10 | |||
| 3 | 1.34 | 1.19 | |||
| 4 | 1.53 | 1.41 | |||
| 5+ | 1.88 | 1.64 | |||
| RIC/NST regimens | 0 | 1.00 | 0.001 | 1.00 | <0.0001 |
| 1 | 0.95 | 1.12 | |||
| 2 | 1.10 | 1.12 | |||
| 3 | 1.27 | 1.27 | |||
| 4 | 1.46 | 1.39 | |||
| 5+ | 1.66 | 1.65 | |||
| Lymphoid diseases | 0 | 1.00 | <0.0001 | 1.00 | <0.0001 |
| 1 | 1.16 | 1.15 | |||
| 2 | 1.24 | 1.12 | |||
| 3 | 1.37 | 1.32 | |||
| 4 | 2.13 | 1.67 | |||
| 5+ | 2.15 | 1.88 | |||
| Myeloid diseases | 0 | 1.00 | <0.0001 | 1.00 | <0.0001 |
| 1 | 1.12 | 1.13 | |||
| 2 | 1.00 | 1.06 | |||
| 3 | 1.25 | 1.14 | |||
| 4 | 1.29 | 1.27 | |||
| 5+ | 1.63 | 1.52 | |||
| . | . | TRM . | OS . | ||
|---|---|---|---|---|---|
| . | HCT-CI scores . | HR . | p-value . | HR . | p-value . |
| High-dose regimens | 0 | 1.00 | <0.0001 | 1.00 | <0.0001 |
| 1 | 1.19 | 1.14 | |||
| 2 | 1.12 | 1.10 | |||
| 3 | 1.34 | 1.19 | |||
| 4 | 1.53 | 1.41 | |||
| 5+ | 1.88 | 1.64 | |||
| RIC/NST regimens | 0 | 1.00 | 0.001 | 1.00 | <0.0001 |
| 1 | 0.95 | 1.12 | |||
| 2 | 1.10 | 1.12 | |||
| 3 | 1.27 | 1.27 | |||
| 4 | 1.46 | 1.39 | |||
| 5+ | 1.66 | 1.65 | |||
| Lymphoid diseases | 0 | 1.00 | <0.0001 | 1.00 | <0.0001 |
| 1 | 1.16 | 1.15 | |||
| 2 | 1.24 | 1.12 | |||
| 3 | 1.37 | 1.32 | |||
| 4 | 2.13 | 1.67 | |||
| 5+ | 2.15 | 1.88 | |||
| Myeloid diseases | 0 | 1.00 | <0.0001 | 1.00 | <0.0001 |
| 1 | 1.12 | 1.13 | |||
| 2 | 1.00 | 1.06 | |||
| 3 | 1.25 | 1.14 | |||
| 4 | 1.29 | 1.27 | |||
| 5+ | 1.63 | 1.52 | |||
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal