Abstract
Abstract  739
739
The combination of a calcineurin inhibitor and methotrexate has been the standard of care in graft-vs.-host disease (GVHD) prophylaxis for over 25 years, with resultant rates of grade II-IV acute GVHD between 30–50%. The mTOR inhibitor, sirolimus, has demonstrated promise in a number of Phase II trials as an immunosuppressant used for GVHD prophylaxis. The BMT CTN, sponsored by the NHLBI and NCI, conducted a multicenter, randomized controlled trial comparing the combination of tacrolimus and sirolimus (Tac/Sir) with tacrolimus and methotrexate (Tac/Mtx) as GVHD prophylaxis after matched, related donor (MRD) hematopoietic stem cell transplantation (HSCT).
Eligible patients were between ages 2 – 60 years, and had acute leukemia in remission, myelodysplasia or chronic myeloid leukemia in chronic or accelerated phase. All had adequate organ function, and a 6/6 HLA-A, B, DRB1 matched sibling donor. 304 patients were randomly assigned to either Tac/Sir (n = 151) or Tac/Mtx (n = 153) as GVHD prophylaxis after TBI-based conditioning and MRD HSCT. An intent-to-treat analysis was performed on the primary endpoint of Grade II-IV GVHD-free survival 114 days from randomization. Ten subjects who received busulfan-based conditioning and were previously reported were excluded from analysis. Three subjects who did not undergo HSCT are included in the primary analysis, but not secondary analyses.
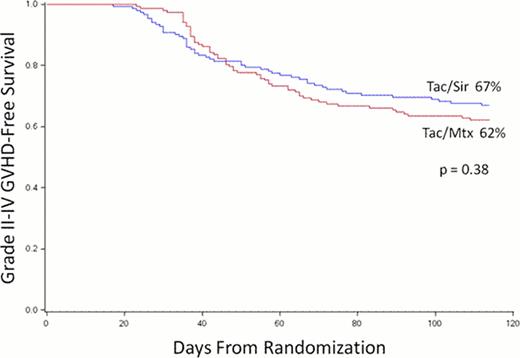
Treatment groups were well balanced. The median age of participants was 44 years (range 13 – 59) and 83% had acute leukemia. Neutrophil and platelet engraftment were both faster in the Tac/Sir group (14 vs. 16 days, p < 0.001; 16 vs. 19 days, p = 0.03, respectively), but this did not affect the time to first hospital discharge (20 vs. 21 days, p = 0.37). The incidence of grade II-IV and grade III-IV acute GVHD at 100 days were lower in the Tac/Sir group (26 vs. 34%, p = 0.17; 8 vs. 15%, p = 0.05). Day 100 treatment-related mortality was no different between groups (7 vs. 7%, p = 0.43). The primary endpoint of 114-day acute GVHD-free survival was not statistically different between groups (67 vs. 62%, p = 0.38, Figure). The cumulative incidence of relapse at 2 years from transplantation was not different between groups (27 vs. 30%, p = 0.81). The competing-risk cumulative incidence of chronic GVHD was higher in the Tac/Sir arm (54 vs. 43%, p =0.044). Overall toxicities were not different between groups, with two notable exceptions. The peak and average OMAS oral mucositis scores were lower in the Tac/Sir arm (peak 0.70 vs. 0.96, p < 0.001; average 0.31 vs. 0.47, p < 0.001), however, there was an increased rate of the endothelial injury syndromes, veno-occlusive disease (11 vs. 4%, p = 0.03), and thrombotic microangiopathy (5 vs. 1%, p = 0.05) in the Tac/Sir arm. Causes of death were not different between groups. At 2 years from transplantation, disease-free (DFS) and overall survival (OS) were not different between study arms (DFS 53 vs. 53%, p = 0.76; OS 60 vs. 61%, p = 0.44).
No difference in 114-day acute GVHD-free survival was noted between treatment arms. Compared with Tac/Mtx in MRD HSCT, Tac/Sir is associated with more rapid engraftment, less severe acute GVHD and oral mucositis, excess chronic GVHD and endothelial injury syndromes, and similar long-term outcomes. Understanding the trade-offs between regimens, Tac/Sir can be used as an alternative to Tac/Mtx in MRD HSCT.
Cutler:Pfizer, inc: Research Funding; Astellas, Inc: Consultancy, Research Funding. Off Label Use: Sirolimus - Prevention of GVHD Tacrolimus - Prevention of GVHD. Waller:Outsuka: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal