Abstract
Abstract  742
742
AZA has immunomodulatory properties that may affect donor lymphocytes favorably, potentially leading to less GVHD after HSCT. We have been investigating low-dose AZA (32 mg/m2 daily for 5 days) to prevent AML/MDS relapse after HSCT. Interestingly, in our dose finding phase I study (Cancer, 2010) there was a suggestion of less cGVHD with longer AZA treatments. We then hypothesized that this approach leads to less cGVHD, and performed a comparison of patients that received AZA to prevent relapse versus historic controls that did not receive the drug. Major objective of this analysis is to determine the cumulative incidence of cGVHD with versus without AZA.
Patients received AZA based on high risk of relapse disease, starting at a median of 45 days from transplant (range, 17–149). AZA effect on aGVHD was not studied since the drug was started after most cases of aGVHD had already occurred or/and had resolved or improved. Patients with active acute GVHD (aGVHD) were not eligible to receive AZA. Median dose was 32 mg/m2 (range, 8–40). Median number of AZA cycles was 3 (range, 1–54), and median time on AZA for patients that received >3 cycles (n=37) was 144 days (range, 93–1329). Using a computer algorithm, we randomly selected from our departmental database a control group consisting of patients who had received HSCT within the same time period, and had similar GVHD prophylaxis, stem cell source, and comparable low risk of grade II-IV aGVHD (Table). Two hundred and thirty patients were identified fulfilling these criteria. The rate of cGVHD was compared between the AZA group (grouped as 1–3 cycles and >3 cycles) and the control group, in a landmark analysis starting at 6 months after HSCT. Leukemia relapse or death in remission before cGVHD onset were considered as competing risks in this analysis.
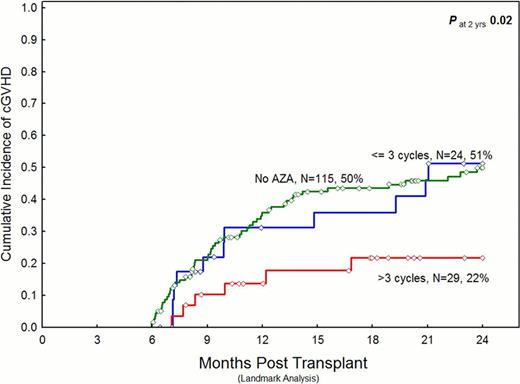
Median follow-up was 25 months (range, 1–99) in the AZA group, and 31 months (range, 1.5–117) in the control group. 29, 24, and 115 patients in the >3 AZA cycles, 1–3 AZA cycles, and the control group were evaluable for the 6-month landmark analysis, respectively. The numbers of patients developing cGVHD and the HR at 2 years in this analysis were as follows: 53 of 115 controls (reference group), 11 of 24 patients who received 1–3 AZA cycles (HR at 2 years, 0.9; P=NS), and 6 of 29 patients who received >3 AZA cycles (HR at 2 years, 0.4; 95% confidence interval (CI), 0.1–0.8; P=0.02) developed cGVHD. Similarly, in a landmark analysis, the cumulative incidence of cGVHD was significantly lower in the subgroup that received >3 AZA cycles (figure).
Low-dose AZA appears to reduce the likelihood of developing cGVHD. We are investigating if this effect is associated with preservation or improvement of the graft-versus-leukemia effect in an ongoing, randomized study.
| . | Controls (n=230) . | AZA<=3 cycles (n=48) . | AZA>3 cycles (n=37) . | P (controls X AZA>3 cycles . |
|---|---|---|---|---|
| Median age | 52 | 60 | 52 | 0.9 |
| Ablative preparative regimen | 63% | 25% | 30% | <0.001 |
| Disease status (remission (CR1/CR2)/ active disease) | 64%/36% | 31%/69% | 41%/59% | 0.01 |
| AML/MDS | 95% | 96% | 86% | 0.07 |
| Second allo HSCT | 7% | 15% | 22% | 0.01 |
| Peripheral blood graft | 86% | 75% | 70% | 0.02 |
| <10/10 HLA match | 4% | 11% | 13% | P=NS |
| Tacrolimus-based GVHD prophylaxis | 98% | 92% | 89% | 0.01 |
| aGVHD incidence (grade II-IV/III-IV) | 10%/2% | 17%/11% | 25%/3% | 0.01 (gd II-IV) |
| . | Controls (n=230) . | AZA<=3 cycles (n=48) . | AZA>3 cycles (n=37) . | P (controls X AZA>3 cycles . |
|---|---|---|---|---|
| Median age | 52 | 60 | 52 | 0.9 |
| Ablative preparative regimen | 63% | 25% | 30% | <0.001 |
| Disease status (remission (CR1/CR2)/ active disease) | 64%/36% | 31%/69% | 41%/59% | 0.01 |
| AML/MDS | 95% | 96% | 86% | 0.07 |
| Second allo HSCT | 7% | 15% | 22% | 0.01 |
| Peripheral blood graft | 86% | 75% | 70% | 0.02 |
| <10/10 HLA match | 4% | 11% | 13% | P=NS |
| Tacrolimus-based GVHD prophylaxis | 98% | 92% | 89% | 0.01 |
| aGVHD incidence (grade II-IV/III-IV) | 10%/2% | 17%/11% | 25%/3% | 0.01 (gd II-IV) |
de Lima:Celgene: Research Funding. Off Label Use: azacitidine: off-label use as maintenance therapy following allogeneic stem cell transplant for MDS/AML. garcia Manero:celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal