Abstract
Antibody-forming cells (AFCs) expressing the chemokine receptor CXCR3 are recruited to sites of inflammation where they help clear pathogens but may participate in autoimmune diseases. Here we identify a mechanism that induces CXCR3 expression by AFC and germinal center (GC) B cells. This happens when CD8 T cells are recruited into CD4 T cell–dependent B-cell responses. Ovalbumin-specific CD4 T cells (OTII) were transferred alone or with ovalbumin-specific CD8 T cells (OTI) and the response to subcutaneous alum-precipitated ovalbumin was followed in the draining lymph nodes. OTII cells alone induce T helper 2-associated class switching to IgG1, but few AFC or GC B cells express CXCR3. By contrast, OTI-derived IFN-γ induces most responding GC B cells and AFCs to express high levels of CXCR3, and diverse switching to IgG2a, IgG2b, with some IgG1. Up-regulation of CXCR3 by GC B cells and AFCs and their migration toward its ligand CXCL10 are shown to depend on B cells' intrinsic T-bet, a transcription factor downstream of the IFN-γR signaling. This model clarifies how precursors of long-lived AFCs and memory B cells acquire CXCR3 that causes their migration to inflammatory foci.
Introduction
Homeostatic chemokines, including CXCL13, CXCL19/CXCL21, and CXCL12, are essential organizers of lymphoid tissues in steady states. For instance, they govern the compartmentalization between T and B cells. Importantly, they are also involved in the repositioning of these lymphocytes during the different stages of immune responses. The development of protective memory B cells and long-lived AFCs relies in part on changing expression by the responding B cells of the receptors for the chemokines CXCR5, CCR7, and CXCR4, and of the orphan receptor Epstein-Barr virus-induced molecule-2.1-3 Thus, during the development of T-dependent antibody responses to protein-based antigens, such as alum-precipitated protein vaccines, chemokine-driven movements in lymph nodes (LNs) are sequentially involved in: (1) the cognate interaction of activated B cells with primed CD4 T cells4-6 in the outer T zone, resulting in B-cell proliferation and class-switch recombination (CSR); or (2) the signals that determine whether B blasts differentiate outside follicles into AFCs without going through affinity maturation1,3 or form germinal center (GC) in follicles.2 Through CXCR4- and CXCR5-dependent movements, B cells undergo affinity maturation in GC through proliferation, hypermutation of their immunoglobulin (Ig) variable region genes, and selection of high-affinity mutants that emerge as memory B cells or long-lived AFCs.7,8 The acquisition of CXCR4 by AFC attracts them to CXCL12 produced in bone marrow's long-term survival niches. In these niches, they maintain protective antibody titers over months.9,10
Alongside the varying expression of CXCR5/CCR7/CXCR4 that modulates B-cell chemotaxis toward homeostatic chemokines, inflammatory conditions can induce IFN-γ–dependent expression of CXCR3 by lymphocytes, including AFCs. This receptor confers responsiveness to CXCL9, CXCL10, and CXCL11, which are produced at high levels in sites of inflammation.11
This CXCR3-dependent pathway is important for the recruitment of lymphocytes at sites of infection and clearance of pathogens.12,13 For instance, CXCR3 expression by mouse AFCs is critical for their migration to the CNS during viral encephalomyelitis. Thus, CXCR3-dependent migration of AFCs to the site of infection clears the virus from the CNS, although this is not achieved with systemic release of antibody.14,15
CXCR3+ AFCs and memory B cells were found at particularly high frequency in peripheral blood of patients with autoimmune diseases mediated by autoantibodies.16,17 This suggests that self-reactive AFCs may be produced and/or attracted to and sustained in chronic inflammatory niches through a mechanism that involves CXCR3 and its ligands.18 In mouse models for lupus erythematosus, AFCs are found in inflamed tissues.19 Knockdown of CXCR3 has shown the importance of this chemokine receptor in the development of the autoimmune disorders,20 including the production anti–double-stranded DNA IgG1.21
Despite the importance of CXCR3 induction for antibody production in inflammatory foci, the mechanism by which CXCR3 is acquired by AFCs and their precursors in GC has not been characterized. We have recently shown that antigen-specific CD8 T cells produce IFN-γ and induce IFN-γ production by CD4 T helper cells in draining LN of mice immunized with alum-precipitated protein.22,23 We now test whether this response favors CXCR3 expression by plasmablasts and GC B cells and, if so, how this expression is regulated. The likely role of IFN-γ in this response led us to probe the requirement of the T cell–specific T-box transcription factor (T-bet) in responding B cells. T-bet was first identified as a regulator active in T helper 1 type responses and necessary for CD4 T cells to produce IFN-γ.24 T-bet plays a differential role in IFN-γ-induced CSR, for switching to IgG2a induced by this cytokine requires T-bet within the B cells whereas IFN-γ-induced CSR to IgG2b is T-bet-independent.23 The present report points to a key requirement for B cell T-bet for antibody production in inflammatory foci.
Methods
Mice, adoptive transfer, immunization, and IFN-γ blockade
Wild-type (WT) CD45.2+ C57BL/6J mice were purchased from HO Harlan OLAC. OTII mice transgenic for αβTCR specific for 323-339 ovalbumin (OVA)–peptide (ISQAVHAAHAEINEAGR) in the context of H-2 I-Ab, and OTI mice transgenic for αβTCR specific for OVA-peptide 257-264 (SIINFEKL) in the context of H-2Kb (Charles River) were crossed to CD45.1+ C57BL/6J congenic mice (The Jackson Laboratory). B1.8hi mice were kindly supplied by Dr Michel C. Nussenszweig (Rockefeller University, New York, NY); these mice carry prerearranged VHDJH genes specific for the hapten 4-hydroxy-3-nitrophenyl acetyl (NP) when combined to Igλ light chain.25 CD45.1+ B1.8hi cells were obtained by crossing CD45.1+ mice deficient for Igκ light chain with B1.8hi mice. For adoptive transfer, CD4 T cells from LNs of OTII mice were purified using anti-CD4 MACS microbeads, CD8 T cells from LN of OTI mice were purified using anti-CD8 MACS microbeads, and B cells from LN of B1.8hi Igk−/− mice were purified using anti-B220 MACS microbeads (Miltenyi Biotec). The different types of chimeras were constructed by injecting intravenously 2 × 106 purified CD45.1+OTI, 2 × 106 purified CD45.1+OTII, and 106 purified CD45.1+B1.8hi cells into WT or T-bet−/− (The Jackson Laboratory) congenic CD45.2+ recipient mice. The chimeras were immunized with alum-precipitated ovalbumin (alumOVA), or where stated, alum-precipitated NP-OVA conjugate (alumNP-OVA). These were respectively prepared by mixing endotoxin-free OVA protein (Hyglos) or NP-conjugated ovalbumin (Biosearch Technologies) with a 9% aluminum potassium sulfate (Sigma-Aldrich) solution. A total of 10 μg of alumOVA or alumNP-OVA in a final volume of 10 μL in PBS was injected subcutaneously into both footpads. In vivo IFN-γ neutralization was achieved by giving, at the time of immunization and 3 days later, 2 intravenous injections of 1 mg of rat anti–IFN-γ antibody clone XGM1.2 (BIO X CELL). Control mice were similarly given rat IgG1 clone HRPN antibody (BIO X CELL). All animals were maintained under standard animal house conditions following local and United Kingdom Home Office regulations.
Flow cytometric analysis and FACS cell sort
LN single-cell suspensions were prepared in RPMI medium containing 5% FCS, 0.15 mg/mL DNase I (Sigma-Aldrich) and incubated for 5 minutes with 10mM EDTA (Sigma-Aldrich). Cells were then treated for 15 minutes on ice with supernatant from 2.4G2 hybridoma culture and 5% normal mouse serum in FACS buffer (2mM EDTA-PBS supplemented with 0.1% FCS). The antibodies used for surface staining and intracellular staining were: FITC-conjugated anti–mouse CD45.1 (clone A20, eBioscience), anti–mouse GL7 (BD Biosciences PharMingen), goat polyclonal antibodies against mouse IgM, IgG1, IgG2a, and IgG2b (Southern Biotechnology); PE-conjugated anti–mouse CD138 (clone 281-2) from BD Biosciences PharMingen, and anti–mouse CXCR3 (clone 220803) from R&D System; peridinin chrorophyll protein tandem Cy5.5-conjugated anti–mouse B220 (clone RA3-6B2 BD Biosciences PharMingen); allophycocyanin-conjugated anti–mouse CD138 (clone 281-2) streptavidin from BD Biosciences PharMingen; biotin-conjugated anti–mouse CD95 (clone Jo2; BD Biosciences PharMingen). Intracellular staining was performed using Cytofix/cytoperm kit (BD Biosciences). Cells were sorted using a MoFlo cell sorter (Dako), and analysis by flow cytometry of LN cell suspensions, including purity assessment of the sorted cells, was done using a FACScalibur (BD Biosciences). Final analyses and graphical output were performed using FlowJo Version 8.2 software (TreeStar). The numbers of AFCs and GC B cells per LN were calculated by counting manually the total number of cells per LN and reporting this number to the percentage of AFCs and GC B cells found in the LN.
ELISPOTS
A total of 600 FACS-sorted CD138+B220int AFCs obtained from pooled mesenteric, popliteal, brachial, and inguinal LN from OTII + OTI nonimmunized or alum-immunized chimeras, or 600 CD138+B220intCXCR3−, or CXCR3+ obtained from popliteal LN of immunized OTII-only or OTII + OTI chimeras, respectively, were placed for 3 hours at 37°C on 96-well plate Millipore membranes coated with 100 μg/mL OVA and blocked with 1% BSA (Sigma-Aldrich). After washing, the plates were incubated overnight at 4°C with peroxidase-conjugated goat antimouse IgG (Southern Biotechnology) in 1% BSA/0.05% Tween 20 PBS. After washes, spots were revealed with 3-amino-9-ethyl carbazole at 1.58mM in 0.1M acetate buffer, pH 5, 0.015% H2O2. Pictures were taken with a Leica MZ75 microscope equipped with a Leica 420C digital camera, and dots were counted by eye from the digital pictures.
Real-time PCR
mRNA extraction, reverse transcription, and gene expression by real-time PCR has been described previously.26 PCR was performed on ABI 7900 using TaqMan chemistry (Applied Biosystems). TaqMan probes and primers were designed by using Primer Express Version 3.0 computer software (Applied Biosystems) and synthesized by Eurogenetec. Standard reaction conditions for the TaqMan PCR were used. The sequences for primers and probes are as follows: T-bet: forward primer, 5′-ATGCCAGGGAACCGCTTATA-3′; reverse primer, 5′-AACTTCCTGGCGCATCCA-3′; probe, FAM-conjugated 5′-CCCAGACTCCCCCAACACCGGA-3′; β2-microglobulin: forward primer, 5′-CTGCAGAGTTAAGCATGCCAGTAT-3′; reverse primer 5′-ATCACATGTCTCGATCCCAGTAGA-3′; probe: NED-conjugated 5′-CGAGCCCAAGACC-3′. CXCR3 mRNA was quantified using the assay-on-demand Mm00438259_m1 from Applied Biosystems. Relative quantification of T-bet mRNA was calculated by referring to the β2-microglobulin mRNA levels, quantified in a duplex PCR. mRNA transcript level of each gene was analyzed by using Applied Biosystem SDS Version 2.3 software by setting thresholds determining the cycle number at which the threshold was reached (Ct). The Ct of the β2-microglobulin was subtracted from the Ct of the target gene, and the relative amount was calculated as 2−ΔCt.
Migration assay
Cell suspensions of the popliteal LN from the different types of immunized chimeras indicated in Figure 6 were prepared in RPMI medium containing 1% BSA. A total of 2 million cells were plated in the top chamber of 6.5-mm diameter, 5-μm pore polycarbonate TransWell culture insert (Corning Life Sciences). The bottom chamber contained 300 ng/mL of CXCL10 or 300 ng/mL of CXCL12 (PreproTech), or medium. After 2 hours of incubation at 37°C, cells from the top and the bottom chambers of each well were stained for analysis by flow cytometry. The number of cells in each sample was counted by FACS using AccuCheck Counting Beads (Invitrogen) as reference. The migration index for each cell suspension was calculated by dividing the average numbers of cells that migrated in the triplicates containing CXCL10 in the bottom chamber, by the average number of cells of the same population that passed nonspecifically by gravitation to the bottom chamber containing medium only.
Results
OVA-specific CD8 (OTI) T cells induce CXCR3 expression by AFCs and GC B cells in responses to alumOVA
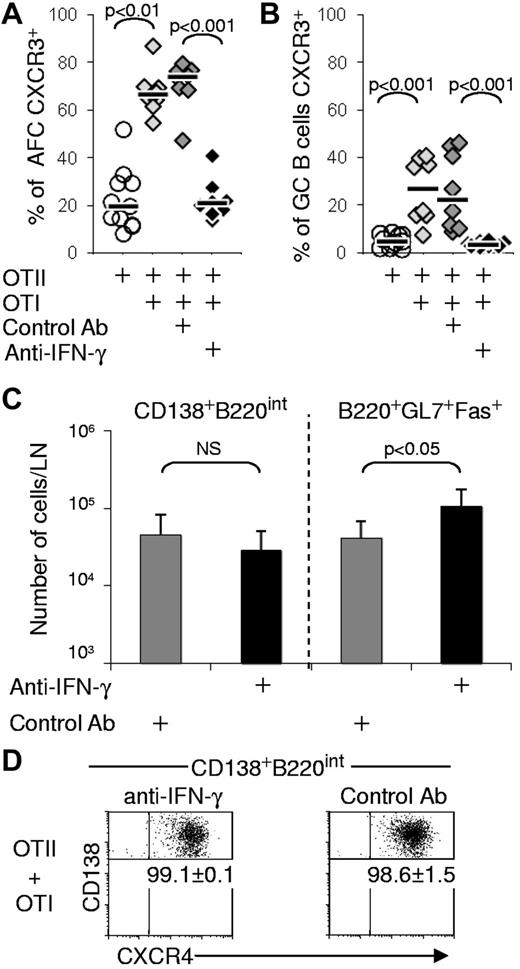
To test whether antigen-specific CD8 T cells can induce responding B cells to express the chemokine receptor CXCR3, we used chimeras constructed by the transfer of OVA-specfic CD4 (OTII) cells only or in conjunction with OVA-specific CD8 (OTI) T cells into congenic WT mice. The chimeras were then immunized in the footpads with endotoxin-free alumOVA or left nonimmunized. The response of the endogenous B cells in the draining popliteal LN was assessed 7 days later in all types of chimeras. In chimeras with OTI and OTII cells and immunized with alumOVA, both CD138+B220Intermediate(Int) AFC and B220+GL7+Fas+ GC B cells up-regulated CXCR3 substantially more frequently and to a greater extent than in chimeras constructed with OTII cells only (Figure 1A-B). By contrast, CXCR4 up-regulation by AFCs and GC B cells was comparable in the 2 sets of chimeras (Figure 1A). The total numbers of CD138+B220Int AFC produced per LN were comparable in both OTII-only and OTII + OTI chimeras, whereas nonimmunized OTII + OTI chimeras had fewer than one-thousandth of the number of AFCs found in the immunized nodes (Figure 1C left). ELISPOT using OVA as the target antigen show that both CXCR3− and CXCR3+ AFCs isolated from popliteal LN of OTII only or OTII + OTI chimeras were OVA-specific (Figure 1D). By contrast, none of the few AFCs from the LN from nonimmunized or alumPBS immunized OTII + OTI chimeras were OVA-specific. Thus, the AFCs induced in popliteal LN of alumOVA-immunized mice are OVA-specific. Substantial GCs are formed in the OTII + OTI chimeras, although the numbers of GC B cells isolated were around half those found in the chimeras constructed with OTII cells alone (Figure 1C right). LN of nonimmunized OTII + OTI chimeras contained fewer than one-thousandth of the number of GC B cells found in the immunized nodes, indicating that GC in OTII or OTII + OTI chimeras developed as the result of alumOVA immunization (Figure 1C right).
OTI cells induce CXCR3 expression on AFCs and GC B cells. Chimeras were constructed in CD45.2+ C57BL6 mice by transfer of CD45.1+ OTII cells or CD45.1+ OTII + OTI cells. These chimeras were immunized with alumOVA or alumPBS in both footpads, or left nonimmunized (NI). Seven days after immunization, cell suspensions were prepared from the 2 popliteal LN. The expression of CXCR3 and CXCR4 was assessed by flow cytometry on CD138+B220int AFCs (A) and B220+GL7+Fas+ GC B cells (B). The numbers on FACS plots represent the percentages of AFC (A) or GC B cells (B) expressing CXCR3 or CXCR4. (C) Geometric mean ± SD of CD138+B220int AFCs (left) and B220+GL7+Fas+ CG B cells (right) per popliteal LN in the different types of chimeras. These data are derived from 2-5 independent experiments with a total of 4-12 mice per group. (D) OVA specificity of FACS-sorted CXCR3− or CXCR3+ AFCs isolated from, respectively, OTII-only or OTII + OTI chimeras day 7 after alumOVA, or of total AFCs from OTII + OTI chimeras NI or at 7 days after alumPBS in footpads was assessed by ELISPOT. Mean ± SD are representative of 3 independent experiments involving 6 mice per group. In both OTII-only or OTII + OTI chimeras immunized with alumOVA, one-third of the plated AFCs could be detected as OVA-specific, although none of the AFCs sorted from NI or alum-immunized OTII + OTI chimeras were detected as OVA-specific. The significance of differences is assessed by 2-tailed Mann-Whitney nonparametric statistics. NS indicates not significant.
OTI cells induce CXCR3 expression on AFCs and GC B cells. Chimeras were constructed in CD45.2+ C57BL6 mice by transfer of CD45.1+ OTII cells or CD45.1+ OTII + OTI cells. These chimeras were immunized with alumOVA or alumPBS in both footpads, or left nonimmunized (NI). Seven days after immunization, cell suspensions were prepared from the 2 popliteal LN. The expression of CXCR3 and CXCR4 was assessed by flow cytometry on CD138+B220int AFCs (A) and B220+GL7+Fas+ GC B cells (B). The numbers on FACS plots represent the percentages of AFC (A) or GC B cells (B) expressing CXCR3 or CXCR4. (C) Geometric mean ± SD of CD138+B220int AFCs (left) and B220+GL7+Fas+ CG B cells (right) per popliteal LN in the different types of chimeras. These data are derived from 2-5 independent experiments with a total of 4-12 mice per group. (D) OVA specificity of FACS-sorted CXCR3− or CXCR3+ AFCs isolated from, respectively, OTII-only or OTII + OTI chimeras day 7 after alumOVA, or of total AFCs from OTII + OTI chimeras NI or at 7 days after alumPBS in footpads was assessed by ELISPOT. Mean ± SD are representative of 3 independent experiments involving 6 mice per group. In both OTII-only or OTII + OTI chimeras immunized with alumOVA, one-third of the plated AFCs could be detected as OVA-specific, although none of the AFCs sorted from NI or alum-immunized OTII + OTI chimeras were detected as OVA-specific. The significance of differences is assessed by 2-tailed Mann-Whitney nonparametric statistics. NS indicates not significant.
CXCR3 expression by AFCs and GC B cells induced by CD8 T cells is largely mediated through IFN-γ
We next tested whether the induction of CXCR3 expression on AFCs and GC B cells in the chimeras constructed with OTII + OTI cells in response to alumOVA results from IFN-γ production. OTII-only or OTII + OTI chimeras were constructed and immunized as before, but 2 groups of OTII + OTI chimeras received either IFN-γ neutralizing antibody, or a nonbinding control antibody on days 0 and 3 after immunization. At day 7, the percentages of AFCs and GC B cells expressing CXCR3 were dramatically lower in the OTII + OTI chimeras treated with the anti–IFN-γ antibody compared with those in chimeras receiving control antibody or were untreated (Figure 2A-B). Thus, IFN-γ is a major determinant for the acquisition of CXCR3 expression by B cells during a primary LN response to alumOVA. The anti–IFN-γ antibody selectively reduced CXCR3 expression without affecting the up-regulation of CXCR4 (Figure 2D). The neutralization of IFN-γ did not significantly affect the total numbers of CD138+B220Int AFCs produced (Figure 2C left), but caused a significant 2-fold increase in the number of GC B cells (Figure 2C right). This increase in the number of GC B cells caused by IFN-γ neutralization reverses the reduction in GC B-cell numbers seen in chimeras constructed with OTI cells as well as OTII cells (Figure 1C).
CXCR3 expression on AFCs and GC B cells by responding CD8 T cells is induced by IFN-γ. Chimeras were constructed by transfer of OTII or both OTI and OTII cells in C57Bl/6 recipients and were immunized with alumOVA in the footpads 1 day later. Neutralizing anti–IFN-γ antibody or isotype control antibody was given to 2 groups of OTI and OTII chimeras at the time of immunization and 3 days later. Seven days after immunization, the percentage of CXCR3+ cells in the draining LN was assessed by flow cytometry for: (A) CD138+B220int AFC or (B) B220+GL7+Fas+ GC B cells. (C) The geometric mean ± SD of CD138+B220int AFC or B220+GL7+Fas+ CG B cells per popliteal LN in the different types of chimeras is shown. Data are derived from 2 independent experiments with a total of 7 or 8 mice per groups. The significance of differences is assessed by 2-tailed Mann-Whitney nonparametric statistics. NS indicates not significant. (D) Numbers on dot plots indicate the percentage of AFCs expressing CXCR4 ± SD for the groups of immunized WT chimeras that had received OTII + OTI cells and were treated with anti–IFN-γ neutralizing antibody or control antibody. The significance of differences between groups is assessed by 2-tailed Mann-Whitney nonparametric statistics.
CXCR3 expression on AFCs and GC B cells by responding CD8 T cells is induced by IFN-γ. Chimeras were constructed by transfer of OTII or both OTI and OTII cells in C57Bl/6 recipients and were immunized with alumOVA in the footpads 1 day later. Neutralizing anti–IFN-γ antibody or isotype control antibody was given to 2 groups of OTI and OTII chimeras at the time of immunization and 3 days later. Seven days after immunization, the percentage of CXCR3+ cells in the draining LN was assessed by flow cytometry for: (A) CD138+B220int AFC or (B) B220+GL7+Fas+ GC B cells. (C) The geometric mean ± SD of CD138+B220int AFC or B220+GL7+Fas+ CG B cells per popliteal LN in the different types of chimeras is shown. Data are derived from 2 independent experiments with a total of 7 or 8 mice per groups. The significance of differences is assessed by 2-tailed Mann-Whitney nonparametric statistics. NS indicates not significant. (D) Numbers on dot plots indicate the percentage of AFCs expressing CXCR4 ± SD for the groups of immunized WT chimeras that had received OTII + OTI cells and were treated with anti–IFN-γ neutralizing antibody or control antibody. The significance of differences between groups is assessed by 2-tailed Mann-Whitney nonparametric statistics.
The up-regulation of CXCR3 in AFCs induced by CD8 T cells is independent of the pattern of CSR
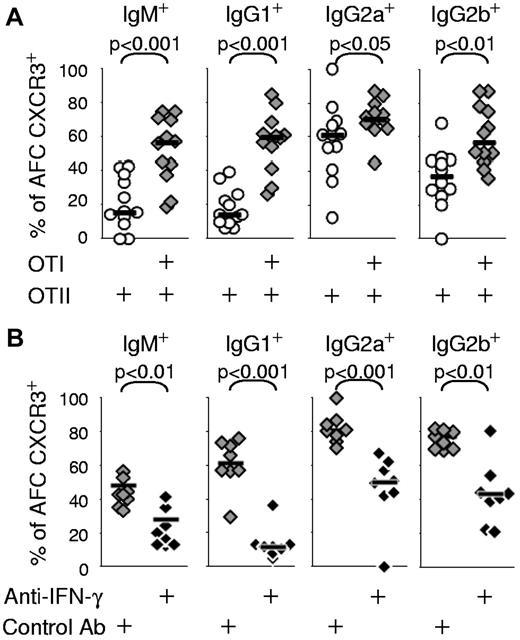
CSR in chimeras constructed with OTII cells is mainly to IgG1, whereas mixed OTII + OTI switch mainly to IgG2a and IgG2b with fewer cells switching to IgG1.23 In addition, treatment with neutralizing anti-IFN-γ changes the switching pattern in mixed OTII + OTI cell chimeras toward that seen in chimeras constructed with OTII cells only.23 Despite the reduction in CSR to IgG1 in mixed chimeras, many of those AFCs in these chimeras that do switch to IgG1 are still induced to express CXCR3 along with the AFCs producing IgG2a or IgG2b or IgM (Figure 3A). Blockade with anti–IFN-γ antibody also reduces expression of CXCR3 in AFCs producing any 1 of these 3 IgG isotypes as well as those producing IgM (Figure 3B). These results suggest that the change of CSR pattern and acquisition of CXCR3 induced by IFN-γ represent independent events, although they may share the same IFN-γ signaling pathway for their induction.
Increase of IFN-γ can induce CXCR3 expression on AFCs of all Ig isotypes. (A) Chimeras constructed by transfer of OTII cells or both OTI and OTII cells were immunized with alumOVA in both footpads. Seven days after immunization, suspensions of cells from the 2 popliteal LN of each mouse were assessed by flow cytometry to determine the proportions of CD138+B220int AFCs of each Ig isotype that expressed CXCR3. The data are derived from 2-5 independent experiments with a total of 8-12 mice in each group. (B) Chimeras were constructed with OTI and OTII cells and immunized in the presence of neutralizing anti–IFN-γ antibody or isotype control antibody, as explained in Figure 2. Seven days after immunization, the proportions of CD138+B220int AFCs of each Ig isotype that expressed CXCR3 were compared by flow cytometry in the draining LNs of the chimeras treated with anti–IFN-γ or with isotype control antibody. Data are derived from 2 independent experiments with a total of 8 mice per group. The significance of differences between groups is assessed by 2-tailed Mann-Whitney nonparametric statistics.
Increase of IFN-γ can induce CXCR3 expression on AFCs of all Ig isotypes. (A) Chimeras constructed by transfer of OTII cells or both OTI and OTII cells were immunized with alumOVA in both footpads. Seven days after immunization, suspensions of cells from the 2 popliteal LN of each mouse were assessed by flow cytometry to determine the proportions of CD138+B220int AFCs of each Ig isotype that expressed CXCR3. The data are derived from 2-5 independent experiments with a total of 8-12 mice in each group. (B) Chimeras were constructed with OTI and OTII cells and immunized in the presence of neutralizing anti–IFN-γ antibody or isotype control antibody, as explained in Figure 2. Seven days after immunization, the proportions of CD138+B220int AFCs of each Ig isotype that expressed CXCR3 were compared by flow cytometry in the draining LNs of the chimeras treated with anti–IFN-γ or with isotype control antibody. Data are derived from 2 independent experiments with a total of 8 mice per group. The significance of differences between groups is assessed by 2-tailed Mann-Whitney nonparametric statistics.
CXCR3 expression on AFCs and GC B cells induced by CD8 T cell–derived IFN-γ is T-bet–dependent
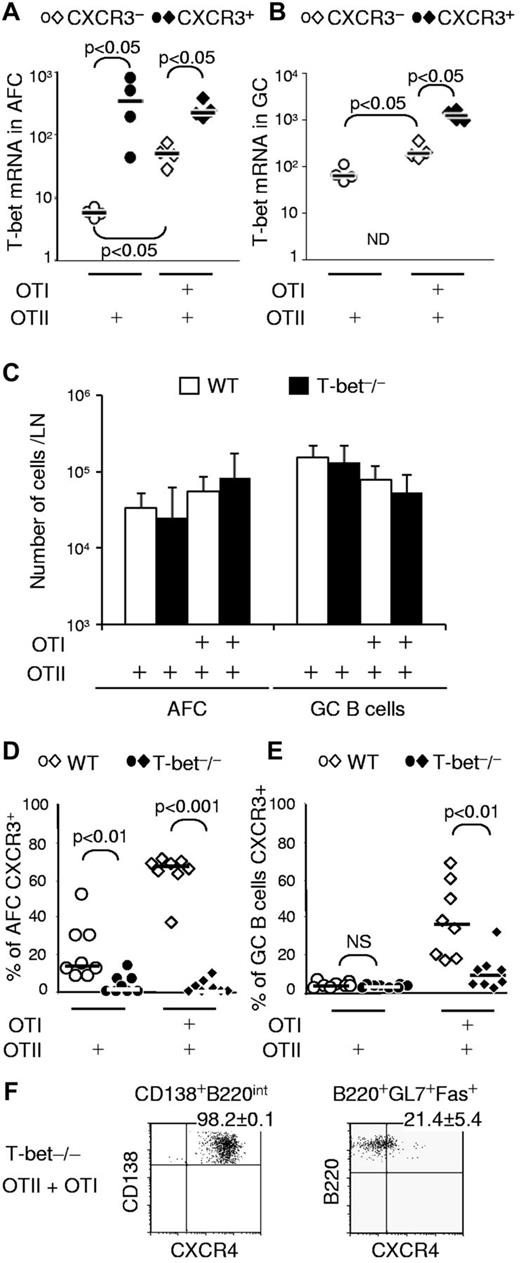
In T cells, IFN-γ–induced CXCR3 expression involves transcriptional regulation by T-bet.27 In addition, we have shown that switching to IgG2a relies on IFN-γ and requires T-bet in B cells.23 To determine whether T-bet plays a role in the induction of CXCR3 expression in B cells responding to alumOVA in mixed chimeras, we FACS-sorted CXCR3− and CXCR3+ AFCs and GC B cells from the draining LN of OTII or OTII + OTI chimeras and quantified the levels of T-bet mRNA by real-time RT-PCR (Figure 4A-B). Levels of T-bet were significantly higher in the CXCR3+ AFCs (Figure 4A) and GC B cells (Figure 4B) than in their CXCR3− counterparts.
IFN-γ-induced CXCR3 expression on AFCs and GC B cells is T-bet–dependent. Chimeras were prepared by transfer of OTII or both OTI and OTII cells. Seven days after immunization with alumOVA, suspensions of cells from the 2 popliteal LNs for each mouse were prepared. (A-B) The level of T-bet mRNA expression was assessed by real-time RT-PCR in (A) CD138+B220int AFCs and (B) B220+GL7+Fas+ GC B cells. ND indicates not determined, as there were almost no CXCR3+ GC B cells. Data are derived from 2 independent experiments with a total of 4 mice per group. (C-E) Further chimeras were constructed by transfer of OTII or both OTI and OTII cells into congenic WT mice or T-bet-deficient mice. Seven days after immunization. (C) Geometric mean ± SD of CD138+B220int AFCs and B220+GL7+Fas+ GC B cells per LN were calculated, and the expression of CXCR3 by (D) CD138+B220int AFCs and (E) B220+GL7+Fas+ GC B cells was assessed by flow cytometry. (F) Numbers on dot plots indicate the percentage of AFC or GC B cells expressing CXCR4 ± SD in T-bet−/− chimeras that had received OTII + OTI cells. Data are derived from 2 independent experiments with a total of 8 mice per group. The significance of differences is assessed by 2-tailed Mann-Whitney nonparametric statistics; only significant differences are indicated.
IFN-γ-induced CXCR3 expression on AFCs and GC B cells is T-bet–dependent. Chimeras were prepared by transfer of OTII or both OTI and OTII cells. Seven days after immunization with alumOVA, suspensions of cells from the 2 popliteal LNs for each mouse were prepared. (A-B) The level of T-bet mRNA expression was assessed by real-time RT-PCR in (A) CD138+B220int AFCs and (B) B220+GL7+Fas+ GC B cells. ND indicates not determined, as there were almost no CXCR3+ GC B cells. Data are derived from 2 independent experiments with a total of 4 mice per group. (C-E) Further chimeras were constructed by transfer of OTII or both OTI and OTII cells into congenic WT mice or T-bet-deficient mice. Seven days after immunization. (C) Geometric mean ± SD of CD138+B220int AFCs and B220+GL7+Fas+ GC B cells per LN were calculated, and the expression of CXCR3 by (D) CD138+B220int AFCs and (E) B220+GL7+Fas+ GC B cells was assessed by flow cytometry. (F) Numbers on dot plots indicate the percentage of AFC or GC B cells expressing CXCR4 ± SD in T-bet−/− chimeras that had received OTII + OTI cells. Data are derived from 2 independent experiments with a total of 8 mice per group. The significance of differences is assessed by 2-tailed Mann-Whitney nonparametric statistics; only significant differences are indicated.
To test whether the increased level of T-bet transcription found in CXCR3-expressing cells was functionally relevant, we assessed whether T-bet–deficient B cells can up-regulate CXCR3. Groups of WT or T-bet–deficient mice were adoptively transferred with OTII cells alone or with OTII + OTI cells. In these chimeras, only the transferred OVA-specific T cells were T-bet sufficient. The chimeras were then immunized as before with alumOVA in the footpads. Seven days after immunization, the endogenous B cells in all of these groups of chimeras whether in WT or T-bet−/− recipients mounted comparable AFC and GC responses (Figure 4C), in keeping with results of experiments reported previously.23 CXCR3 expression was assessed in the AFCs and GC B cells from all chimeras. The absence of T-bet from non–T cells profoundly reduced the capacity to up-regulate CXCR3 in AFCs (Figure 4D). This also applied to the GC B cells, although in the OTII + OTI cell chimeras the loss of CXCR3 expression by GC B cells was not absolute (Figure 4E). As expected, T-bet deficiency was not found to affect the expression of CXCR4 on AFCs (Figure 4F).
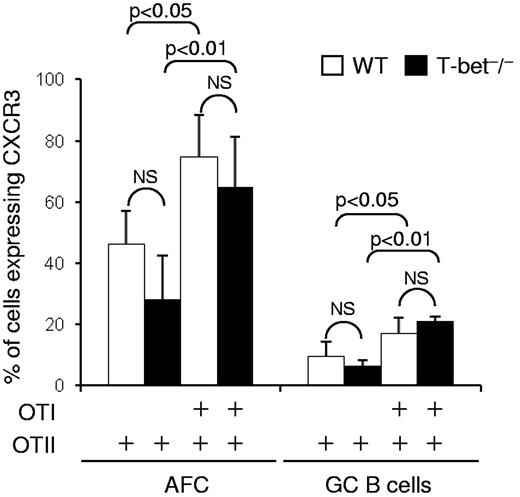
The experiments reported in the previous paragraph (Figure 4C-F) do not exclude an effect of T-bet deficiency in host cells other than B cells. It has been reported that T-bet expression in dendritic cells potentiates the differentiation of naive T cells into IFN-γ–producing Th1 effectors.28 To look for a possible role of T-bet in non–T non–B cells in the induction of CXCR3 in B cells, we constructed further chimeras in which T-bet–sufficient B cells from B1.8hi mice that are specific for the hapten NP were transferred with either OTII or OTII + OTI cells into WT C57BL/6 or congenic T-bet−/− recipients. Seven days after immunization with alumNP-OVA, the proportions of CXCR3+ B1.8hi cells obtained from the T-bet−/− chimeras constructed with OTII + OTI cells were comparable with those seen in WT recipients (Figure 5). Thus, in this system, T-bet in dendritic cells or other non–B non–T cells is dispensable for CD8 T-cell induction of CXCR3 on AFCs and GC B cells. Taken together, this set of experiments identifies an IFN-γ/T-bet–dependent pathway as a key regulator of CXCR3 induction in B cells.
T-bet deficiency in innate cells and nonresponding lymphocytes does not prevent CXCR3 induction in T-bet–sufficient B cells. Chimeras were constructed by transfer of CD45.1+ NP-specific B1.8hi B cells and OTII cells or both OTI + OTII cells either into CD45.2+ WT C57BL/6 or congenic T-bet−/− mice. The chimeras were immunized in both footpads with alumNP-OVA, and 7 days later popliteal LN cell suspensions were prepared as in Figure 1. The percentage of CXCR3+ B1.8hi AFCs (CD138+B220intCD45.1+ cells) and GC B cells (B220+GL7+Fas+ cells) in these suspensions was assessed by flow cytometry. The bar chart represents the mean ± SD (percentage) B1.8hi cells that were CXCR3+. Open bars represent data from WT recipients; and closed bars, data from T-bet−/− recipients. Data are derived from 2 independent experiments with a total of 6 mice in each group. The significance of differences are assessed by 2-tailed Mann-Whitney nonparametric statistics. NS indicates not significant.
T-bet deficiency in innate cells and nonresponding lymphocytes does not prevent CXCR3 induction in T-bet–sufficient B cells. Chimeras were constructed by transfer of CD45.1+ NP-specific B1.8hi B cells and OTII cells or both OTI + OTII cells either into CD45.2+ WT C57BL/6 or congenic T-bet−/− mice. The chimeras were immunized in both footpads with alumNP-OVA, and 7 days later popliteal LN cell suspensions were prepared as in Figure 1. The percentage of CXCR3+ B1.8hi AFCs (CD138+B220intCD45.1+ cells) and GC B cells (B220+GL7+Fas+ cells) in these suspensions was assessed by flow cytometry. The bar chart represents the mean ± SD (percentage) B1.8hi cells that were CXCR3+. Open bars represent data from WT recipients; and closed bars, data from T-bet−/− recipients. Data are derived from 2 independent experiments with a total of 6 mice in each group. The significance of differences are assessed by 2-tailed Mann-Whitney nonparametric statistics. NS indicates not significant.
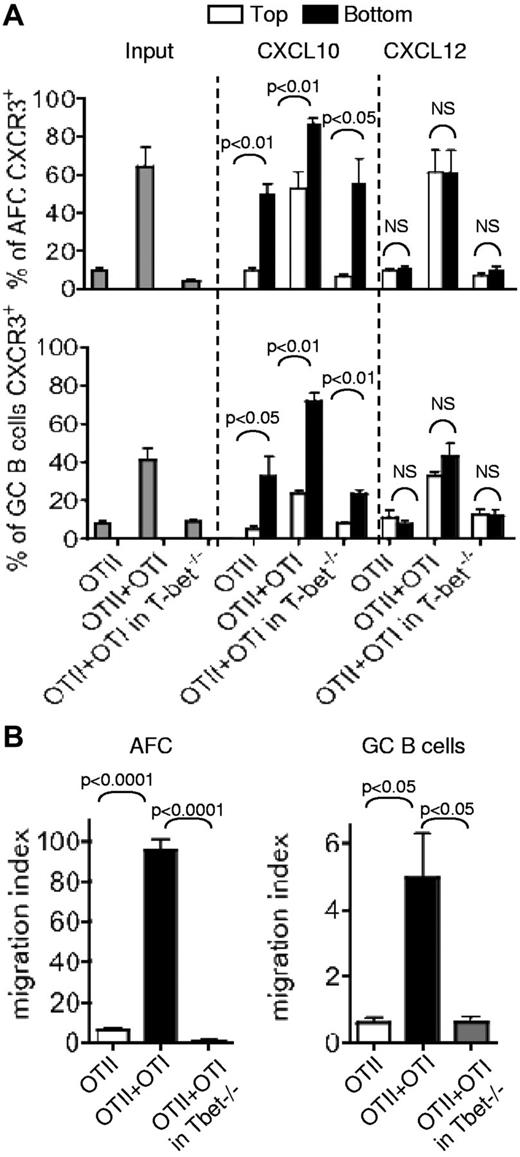
T-bet deficiency impairs CXCR3-mediated attraction of AFCs and GC B cells to CXCL10 in vitro
Previously, it has been shown in vitro that CXCR3 on AFCs promotes their migration toward the 3 ligands for this receptor (CXCL9, CXCL10, and CXCL11),29,30 whereas CXCR4 up-regulated in AFCs or GC B cells promotes their movement toward CXCL12.10,11 To assess the functional significance of CXCR3 on AFCs and GC B cells and the impact of T-bet deficiency on the responsiveness of these cells, we set up a migration assay using CXCL10 or CXCL12. Total LN cells from WT or T-bet−/− recipients that had received OTII cells only or OTII + OTI cells were plated in the upper chambers of transwells containing CXCL10 or CXCL12 in their bottom chambers. After 2 hours of incubation at 37°C, the cells harvested in the top chamber, which had not migrated toward the chemokine and those in the bottom chamber that had migrated were enumerated and stained to identify the proportions of AFCs and GC B cells that expressed CXCR3+ by flow cytometry. The results show that AFCs and GC B cells from the WT chimeras with OTII + OTI cells have the highest proportion of CXCR3+ cells (Figure 6A Input). These cells' respective migration indices (see “Migration assay”) of 95 and 4.9 were significantly higher than those of the AFCs and GC B cells from the WT chimeras transferred with OTII cells only: 6.2 and 0.6 (Figure 6B). T-bet deficiency in the AFCs and GC B cells formed in presence of OTI cells results in a drastic drop in the proportion of CXCR3+ AFCs and GC B cells (Figure 6A gray bars). The migration indices of these cells toward CXCL10 fell accordingly to 0.7 for the AFC and 0.6 for the GC B cells, compared with those of their WT counterparts formed in presence of OTI cells (Figure 6B). There is a significant enrichment in the proportion of CXCR3+ AFCs and GC B cells in the bottom chamber of the transwells containing CXCL10 (Figure 6A middle part of the bar charts). By contrast, there is lack of enrichment in those containing CXCL12 (Figure 6A right part of the bar charts). This indicates that the CXCR3+ cells migrate preferentially toward the specific ligand for this chemokine receptor. Although cell suspensions from T-bet−/− recipients contained only a residual proportion of CXCR3+ AFCs and GC B cells, some specific enrichment in CXCR3+ cells was still visible. Thus, chemotaxis to CXCR3 ligands in AFCs and GC B cells is largely controlled by the level of IFN-γ through the induction of the transcription factor T-bet in these cells or their precursors.
T-bet–dependent migration of CXCR3+ AFCs and GC B cells toward CXCL10. Chimeras were prepared by transfer of OTII cells alone or with OTI cells into congenic WT mice. Other chimeras were constructed by transfer of both OTII and OTI cells into T-bet−/− mice. Seven days after immunization with alumOVA, suspensions of cells from the draining popliteal LN of the different chimeras were prepared. The chemotactic properties of the cells toward CXCL10 (a ligand for CXCR3) or CXCL12 (a ligand for CXCR4) were tested in migration assays using transwells containing 300 ng/mL of chemokine or medium in the bottom chamber. (A) The bar charts represent the percentage of AFCs (top chart) or GC B cells (bottom chart) expressing CXCR3 in the input cell suspensions (gray bars), or the mean ± SD (percentage) in the top chambers (black bars) and in the bottom chambers (white bars) obtained from 2 independent migration assays. The significance of differences was assessed by 2-tailed Mann-Whitney nonparametric statistics. NS indicates not significant. (B) The number of AFCs and GC cells found in the bottom chamber was determined by flow cytometry, and the migration index of the cells was calculated by reference to the number of cells found in the bottom chamber of the control with medium only. The bar charts represent the mean ± SD from triplicates in 1 representative experiment of 3. The figure shows 1 representative experiment of 3 independent experiments. The P values are given for statistically significant differences as calculated by t test.
T-bet–dependent migration of CXCR3+ AFCs and GC B cells toward CXCL10. Chimeras were prepared by transfer of OTII cells alone or with OTI cells into congenic WT mice. Other chimeras were constructed by transfer of both OTII and OTI cells into T-bet−/− mice. Seven days after immunization with alumOVA, suspensions of cells from the draining popliteal LN of the different chimeras were prepared. The chemotactic properties of the cells toward CXCL10 (a ligand for CXCR3) or CXCL12 (a ligand for CXCR4) were tested in migration assays using transwells containing 300 ng/mL of chemokine or medium in the bottom chamber. (A) The bar charts represent the percentage of AFCs (top chart) or GC B cells (bottom chart) expressing CXCR3 in the input cell suspensions (gray bars), or the mean ± SD (percentage) in the top chambers (black bars) and in the bottom chambers (white bars) obtained from 2 independent migration assays. The significance of differences was assessed by 2-tailed Mann-Whitney nonparametric statistics. NS indicates not significant. (B) The number of AFCs and GC cells found in the bottom chamber was determined by flow cytometry, and the migration index of the cells was calculated by reference to the number of cells found in the bottom chamber of the control with medium only. The bar charts represent the mean ± SD from triplicates in 1 representative experiment of 3. The figure shows 1 representative experiment of 3 independent experiments. The P values are given for statistically significant differences as calculated by t test.
Discussion
B cells making primary T-dependent responses to alum-protein vaccines differentiate into GC B cells and AFCs that give rise to switched, affinity matured memory B cells and long-lived postmitotic plasma cells. The features acquired during these primary responses, such as the nature of CSR or the tropism resulting from the induction of specific chemokine receptors or integrins, are likely to impact on the efficacy of recall responses. Previously, in a mouse model using CD4 OTII and CD8 OTI cells specific for OVA, we showed that CD8 T cell–derived IFN-γ could alter the CSR during responses of CD4 T cells and B cells to alumOVA from one typifying Th2 to a Th1 pattern. In the responding B cells, this bias toward Th1 manifests by diversifying the outcome of CSR from the main IgG1 to IgG2a and IgG2b.23 Using the same system, we report now that the level of IFN-γ produced in mixed OTI + OTII cell chimeras also directs the expression of the chemokine receptor CXCR3 on the AFCs or GC B cells formed during these responses. We demonstrate that IFN-γ–induced expression of CXCR3 on AFCs and GC B cells depends on the transcription factor T-bet within the responding B cells. The presence of the OTI CD8 T cells in the mixed chimeras induces higher levels of Ifng mRNA within the OTII cells as well as increasing the proportion of IFN-γ–producing OTII cells.23 As the T cells colonizing GC in this response are only CD4 OTII cells and not CD8 T cells,31 there may well be, in addition to a direct effect of OTI cells on responding B cells, an indirect action through OTII cells that have been influenced by OTI cells.
Interestingly, although CSR to IgG2a also relies on T-bet intrinsic expression in B cells,23 IFN-γ/T-bet–dependent CXCR3 was equally induced on unswitched AFCs and AFCs switched to Ig isotypes that do not rely on T-bet expression for their rearrangements and production, such as IgG1 or IgG2b. Yet, enforced T-bet expression in cell lines or primary cells is sufficient to induce switching to IgG2a.32 The existence of CXCR3+ AFCs that are IgG1+, IgG2b+, or unswitched suggests that T-bet is inducible at different stages of the B-cell differentiation program and is not restricted to IgG2a-switched B cells, as recently proposed.33 Thus, T-bet can regulate independently: (1) the effector functions of the AFCs and of memory B cells by influencing the Ig isotype they produce,23,32 (2) their migratory behavior,14 (3) and possibly, through regulation of CXCR3, the amplitude34 or the longevity33 of antibody responses. This pleiotropic function of T-bet has been also found for CD8,13,24,35 CD4 effector,24,27 or regulatory36 T lymphocytes, NK,24,37,38 and NKT36,39 cells. The present study on B cells adds to the list of the many important functions that T-bet helps to coordinate during IFN-γ-mediated inflammatory responses.
In humans, CXCR3 expression appears to be part of the imprinting of memory B cells. Thus, CXCR3+ memory B cells differentiate into CXCR3+ AFCs on restimulation with CD40L, IL-10, and IL-2, and without the need for IFN-γ.29,30 On the other hand, it has become clear that in mice, CXCR3-dependent migration of AFCs can be critical to their functions in the context of viral infection affecting the CNS, such as those caused by mouse hepatitis virus15 or neurotropic corona virus.14 The acquisition of CXCR3 by post-GC memory B cells through the action of responding CD8 T cells during vaccination with alum-precipitated proteins may be helpful. The resulting rapid induction of CXCR3+ AFC differentiation after challenge may confer protection where systemic release of neutralizing antibody is insufficient and local antibody production is required.
It is not known at this stage whether stimulation of Toll-like receptor 9 on B cells, an IFN-γ–independent molecular pathway that induces T-bet and CSR to IgG2a,40,41 would also induce CXCR3 expression by memory B cells and/or AFCs. It is possible that CpG (hypomethylated cytosine phosphate guanine), a ligand for TLR9 used as a coadjuvant to skew the Th2-biased responses to alum-precipitated proteins,42,43 acts as a substitute of the CD8 T cells for the induction of CXCR3 on responding B cells.
The contribution of T-bet to the development of Th1-dependent autoimmune diseases is well established.32,44 Yet it is not clear whether CXCR3 expressed by autoreactive B cells confers them with migratory properties that enable them to cause harm, either through antibody production or promoting inflammation through cytokine release. The existence of high frequencies of CXCR3+ AFCs or memory B cells in the blood of patients with autoimmune disorders16,17 supports the hypothesis that migration and survival of postmitotic self-reactive AFCs, or plasma cells, are favored at sites of chronic inflammation through a mechanism implicating CXCR3 ligands.18,45 Thus, autoantibodies may be produced by fully differentiated long-lived CXCR3+ plasma cells recruited and persisting in inflamed tissues. Alternatively, in these confined infiltrates, the proximity of IFNγ-producing CD8 T cells, CD4 T cells, and B cells may favor the constant renewal of CXCR3+ short-lived plasma cells from a pool of naive or memory self-reactive B cells.46
In the NZBxNWZ mouse model for lupus, deletion of Cxcr3 in all cells does not reduce cell infiltrates in the kidney or the severity of the disease.21 Surprisingly, absence of CXCR3 in these mice impaired the formation of total and anti–double-stranded DNA autoantibodies of the IgG1 subclass, without affecting the production of IgG2a and IgG2b,21 the typical isotypes generated during T-bet–dependent autoimmune diseases.32 In our previous study, the absence of T-bet in responding B cells and the consequent lack of CXCR3 did not affect the primary T-dependent B-cell response to alum-protein vaccine.23 In addition, the emergence of IgG1 AFCs was only marginally affected.23 This apparent contradiction indicates that impairment of IgG1 switching in CXCR3-deficient NZBxNWZ mice may result from the lack of CXCR3 in T helper cells rather than in B cells. How CXCR3 is required to achieve switching to the typical Th2-associated isotype IgG1 in the context of autoimmune disorders still needs to be clarified. In these complex diseases, a better knowledge of the molecular mechanisms leading to the emergence of potentially harmful cells will help target them through hallmarks acquired during their generation, such as CXCR3 induced in IFN-γ–rich environments.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful for the support of the Biomedical Services Unit at University of Birmingham.
This work was supported by the British Medical Research Council (program grant; I.C.M.M.), a Leverhulme Trust Emeritus Research Fellowship (I.C.M.M.), and MAMOCELL, a NEST project from the European Union (K.-M.T.).
Authorship
Contribution: E.M., K.S., R.E.C., A.C.L., E.H., R.B., and A.F. performed the research; E.M., K.S., I.C.M.M., A.R., A.F.C., K.M., J.D., and R.M. designed the research; K.-M.T. contributed to vital new reagents; E.M., K.S., ACL, I.C.M.M., A.F.C., and R.M. analyzed and interpreted the results; and E.M., K.S., and I.C.M.M. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Karine Serre, Unidade de Imunologia Molecular, Instituto de Medicina Molecular, Av Professor Egas Moniz, 1649-028 Lisboa, Portugal; e-mail: karine@kserre.net; Ian C. M. MacLennan, Institute Biomedical Research, Room 435, University of Birmingham, Birmingham B15 2TT, United Kingdom; e-mail: i.c.m.maclennan@bham.ac.uk; and Elodie Mohr, Instituto Gulbenkian de Ciência, Rua da Quinta Grande 6, Apartado 14, 2781-901 Oeiras, Portugal; e-mail: emohr@igc.gulbenkian.pt.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal