Abstract
The elimination of hepatitis C virus (HCV) in > 50% of chronically infected patients by treatment with IFN-α suggests that plasmacytoid dendritic cells (pDCs), major producers of IFN-α, play an important role in the control of HCV infection. However, despite large amounts of Toll-like receptor 7-mediated IFN-α, produced by pDCs exposed to HCV-infected hepatocytes, HCV still replicates in infected liver. Here we show that HCV envelope glycoprotein E2 is a novel ligand of pDC C-type lectin immunoreceptors (CLRs), blood DC antigen 2 (BDCA-2) and DC-immunoreceptor (DCIR). HCV particles inhibit, via binding of E2 glycoprotein to CLRs, production of IFN-α and IFN-λ in pDCs exposed to HCV-infected hepatocytes, and induce in pDCs a rapid phosphorylation of Akt and Erk1/2, in a manner similar to the crosslinking of BDCA-2 or DCIR. Blocking of BDCA-2 and DCIR with Fab fragments of monoclonal antibodies preserves the capacity of pDCs to produce type I and III IFNs in the presence of HCV particles. Thus, negative interference of CLR signaling triggered by cell-free HCV particles with Toll-like receptor signaling triggered by cell-associated HCV results in the inhibition of the principal pDC function, production of IFN.
Introduction
The eradication of hepatitis C virus (HCV) in > 50% of chronically infected patients by treatment with IFN-α1,2 suggests that production of endogenous IFN-α plays an important role in the control of HCV infection. Plasmacytoid dendritic cells (pDCs) are a highly specialized subset of dendritic cells that function as sentinels for viral infection and are responsible for production of type I (IFN-α and β) and type III (IFN-λ/IL-28/29) IFNs, proinflammatory cytokines, and antigen presentation during viral infection.3-6 pDCs are able to detect genetic material of viruses with a subset of Toll-like receptors (TLRs) localized to the endosomal compartment.7 These nucleotide-sensing TLRs include TLR7, which recognizes single-stranded RNA, and TLR9, which recognizes DNA.
In addition to nucleotide-sensing TLRs, pDCs also recognize pathogens through a battery of cell surface regulatory receptors, including Fc (FcR) and C-type lectin receptors (CLRs). The principal function of these regulatory receptors on pDCs is to facilitate antigen capture and to prevent aberrant immune responses by modulating production of type I IFNs and proinflammatory cytokines.7 Some of these receptors, such as a member of CLR family, blood DC antigen 2 (BDCA-2), associate with the γ-chain of FcϵRI receptor, which contains an immunoreceptor tyrosine-based activation motif. In pDCs, triggering of these receptors initiates a signaling pathway similar to the one that occurs downstream of the B-cell receptor.8 Other CLRs of pDC, such as DC-immunoreceptor (DCIR),9 contain an immunoreceptor tyrosine-based inhibitory motif. Triggering of BDCA-28,10 or DCIR9 inhibits TLR9- and in some cases TLR7-induced production of type I IFNs and proinflammatory cytokines.
Several reports have shown that exposure of pDCs from healthy donors to HCV particles results in no or only weak production of type I IFN.11-15 However, in addition to this passive role, HCV particles also actively impair production of IFN-α triggered in pDCs by synthetic agonists of TLR9.11,13 In contrast to HCV particles, a recent report has shown that HCV-infected hepatoma cells induce in pDCs a robust TLR7-mediated production of type I IFN.14 Here we investigated whether HCV particles suppress production of IFN-α induced in pDCs by HCV-infected hepatoma cells. Furthermore, we investigated the mechanism of the impairment of pDC functions by HCV particles. We demonstrate that, in addition to type I IFNs, exposure of pDCs to HCV-infected hepatoma cells resulted in the production of type III IFNs and that HCV particles or HCV envelope glycoprotein E2 inhibited the production of both types of IFNs by binding to CLRs, BDCA-2 and DCIR. We further show that HCV particles induce in pDCs a rapid phosphorylation of Akt and Erk1/2, in a manner similar to the crosslinking of BDCA-2 or DCIR. Blocking of BDCA-2 and DCIR with Fab fragments of mAbs protects pDCs against the inhibition of IFN production induced by HCV particles. Thus, negative interference of CLR signaling triggered by cell-free HCV particles with TLR signaling triggered by cell-associated HCV results in inhibition of the principal pDC function, production of IFN.
Methods
Isolation and culture of pDCs
PBMCs from healthy anonymous donors were obtained from the national blood services (Etablissement Francais du Sang). pDCs purified from PBMCs as described previously were from 75% to 95% pure, with a contamination of < 5% myeloid dendritic cells.11-13,16 Isolated pDCs were cultured in RPMI 1640 supplemented with 10% FCS and antibiotics. To optimize viability in overnight experiments, recombinant IL-3 (R&D Systems Europe) was added to a final concentration of 10 ng/mL.
Production and purification of cell culture-derived HCVcc (JFH-1 3M)
HCVcc particles were prepared in Huh7.5 cells17 (kindly provided by APATH) on the basis of plasmid pJFH-1 displaying mutations F172C and P173S in core, and N534K in E2,18 as described previously.11 The ultracentrifuged virus purified through a cushion of 20% sucrose was resuspended in RPMI 1640 medium to obtain a 1000-fold concentrated virus suspension containing 107 FFUHuh7.5/1011 HCV RNA copies/mL.
Quantification of viral genome copies
Influenza virus stocks
Influenza virus A/H3N2/Johannesburg/34/99 was produced in MDCK cells.20
Infected and transfected cell lines
Cells of human hepatoma cell line Huh7.5 infected with HCVcc at an MOI of 0.01 focus forming units (FFU)/cell were cultured under standard conditions for 1 week. The percentage of infected cells was determined by immunofluorescence staining for HCV core protein using a mouse monoclonal anticore antibody (C7-50; Thermo Fisher Scientific). Monolayers containing > 60% HCV core+ Huh7.5 cells were used in coculture with pDCs. Huh7.5 cells transfected with H/SG-neo (L + I) subgenomic replicon (SGR; kindly provided by C.M. Rice, Rockefeller University, New York, NY) and virus-free Huh7.5 cells were used as controls.17
Preparation and quantification of HCV E2 glycoprotein
Recombinant His-tagged HCV E2 glycoprotein was prepared as described previously.21,22 It was purified on HisPur Ni-NTA Spin Columns (Thermo Fisher Scientific) according to the manufacturer's instructions. The purified HCV E2 glycoprotein was used as a standard for quantification of HCV E2 glycoprotein in the cell-free supernatants by ELISA. For this purpose, ELISA plates were first coated with 10 μg/mL of RGS-His antibody (QIAGEN). The quantity of recombinant His-tagged HCV E2 glycoprotein was then assessed using a detection anti-E2 antibody AP33 (kindly provided by Genentech) at a concentration 0.4 μg/mL,23 labeled with biotin using EZ-Link Sulfo-NHS-Biotin (Pierce Products, Thermo Fisher Scientific).
Cellular binding of HCVcc or HCV E2 envelope glycoprotein
For the receptor study, COS-7 cells were transiently transfected with human BDCA-2 cloned into pCMV3.0 vector (kindly provided by J. Arthos, National Institute of Allergy and Infectious Diseases, Bethesda, MD)24 or DCIR cloned into a pEGFP-N1 vector (kindly provided by G. J. Adema, Nijmengen Center for Molecular Life Sciences, Nijmengen, The Netherlands)9 using Fugene HD Transfection Reagent (Roche Applied Science) following the manufacturer's instructions.
pDCs or BDCA-2 or DCIR-transfected COS-7 cells were exposed to JFH-1 3M HCV at a multiplicity of 1 up to 100 RNA copies/cell or to recombinant E2 for 30 minutes at 4°C. Unbound virus or E2 glycoprotein was removed by washing of cells with PBS containing 0.9mM CaCl2. Surface expression of BDCA-2 was measured by flow cytometry using BDCA-2-PE (Miltenyi Biotec), DCIR was checked with its GFP tag. HCV particles or soluble HCV E2 (sE2) glycoprotein binding was assessed by flow cytometry using mouse anti-E2 (AP33) labeled with Alexa-647. For the study of JFH-1 3M HCVcc or E2 envelope glycoprotein binding in the presence of antireceptor antibodies, pDCs or COS-7 cells were preincubated 30 minutes at 4°C with mouse anti-DCIR (R&D Systems Europe), anti–BDCA-2 (Miltenyi Biotec), anti-ILT7 (Clinisciences), anti-CD81, anti-CD123 (BD Biosciences), rat anti-CLDN1 (Abnova), and anti–SR-BI or control antibodies (dilution 1/100 or 5 μg/mL).25 After labeling, cells were fixed with 4% paraformaldehyde and analyzed using the LSR II instrument (BD Biosciences) by gating on live Lin−CD11c−CD123+ cells. Data were analyzed with FlowJo Version 7.6.2 software (TreeStar).
Regulatory receptor blocking on pDCs
Fab fragments of anti–BDCA-2 (Miltenyi Biotec) and anti-DCIR (R&D Systems) mAbs were prepared by digestion of antibodies with immobilized ficin for 4 hours at 37°C and by purification on protein A column, using the Pierce Mouse IgG1 Fab Micro Preparation Kit (Perbio). pDCs were incubated with 20 μg/mL anti-BDCA2 and/or anti-DCIR Fab fragments or isotype control in 100 μL culture medium for 30 minutes at 4°C before exposure to HCV particles and further stimulation.
In vitro pDC stimulation
Purified pDCs were kept at a concentration of 106 cells/mL aliquoted in 100-μL quantities in 96-well round-bottom culture plates and stimulated with 10 μg/mL CpG-A (ODN 2216) or CpG-B (ODN 2006; InvivoGen), 5μM R848 (InvivoGen), HCV JFH-1 3M virions at a multiplicity of 100 RNA copies/cell, or Huh7.5 cells infected with HCV JFH-1 3M or transfected with SGR at the ratio of 2 Huh7.5 cell per pDC, for 20 hours in the presence of IL-3.
Determination of Akt and Erk1/2 phosphorylation by dynamic phosphoflow cytometry
Phosphoflow analysis was performed as previously described.26 Briefly, cells were fixed, permeabilized, and incubated successively with Phospho-Akt (Ser473; 193H12) and Erk1/2 (Thr202, Tyr204; 197G2), both rabbit mAb (Cell Signaling) and antirabbit biotinylated antibodies. Finally, detection was performed with a streptavidin-phycoerythrin solution (Beckman Coulter).
Determination of secreted IFN-α and IFN-λ
The quantities of total IFN-α, IFN-λ produced by pDCs were measured in cell-free supernatants using human ELISA kits (IFN-α from eBioscience; IFN-λ [human IL-29/IL-28B, IFN-λ1/3] from R&D Systems Europe).
Statistical analysis
Quantitative variables are expressed as mean ± SEM. To compare the levels of cytokine production by pDCs, we used a Mann-Whitney 2-tailed nonparametric test. Data were analyzed with GraphPad Prism Version 4 software (GraphPad Software). P ≤ .05 was considered to be significant.
Results
HCVcc particles block production of IFN-α in pDCs exposed to HCV-infected hepatoma cells
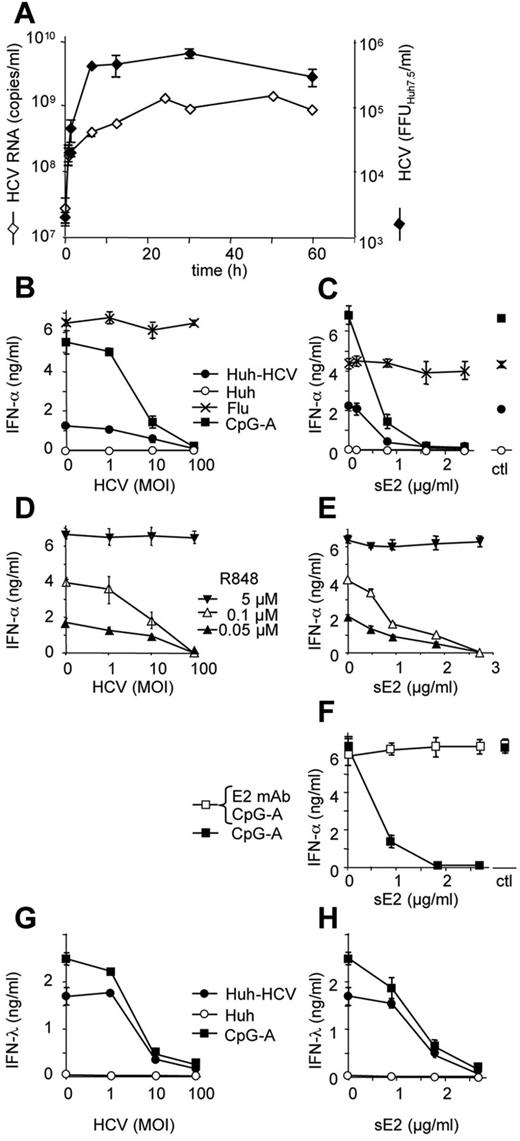
A recent report has shown that pDCs exposed in direct cell-to-cell contact to HCV-infected hepatoma cells produce large amounts of type I IFN via TLR7 signaling.14 We show that, in a standard setup of these coculture experiments, the purified pDCs are mixed with rinsed HCV-infected Huh7.5 cells in a virtual absence of infectious cell-free HCV particles (Figure 1A). The steady-state concentration of infectious HCV particles (4 × 105 FFUHuh7.5/mL) and HCV RNA (109 RNA copies/mL) in the supernatant of HCV-infected Huh7.5 cells was reestablished only 24 hours after initial washing. To simulate conditions in the infected liver, in which pDCs are exposed to both cell-associated and cell-free virus, we mixed purified pDCs with HCV-infected Huh7.5 cells or with control agonists together with defined quantities of HCV particles (JFH-1 3M; Figure 1B). We confirmed that HCV particles did not inhibit TLR7-mediated production of IFN-α stimulated by influenza virus (Figure 1B) or optimal concentration of R848 (Figure 1D).11 However, HCV particles inhibited production of IFN-α stimulated by CpG-A (Figure 1B; P < .05 for MOI ≥ 10) or by suboptimal concentrations of R848 (Figure 1D; P < .05 for MOI ≥ 10). This shows that HCV particles inhibit both TLR7- and TLR9-mediated production of IFN-α. Finally and most importantly, we found that HCV particles disrupted production of IFN-α in pDCs stimulated by the coculture with HCV-infected Huh7.5 cells (Figure 1B; P < .05 for MOI ≥ 10).
HCVcc particles and HCV E2 glycoprotein block production of IFN-α and IFN-λ induced by exposure of pDCs to HCV-infected Huh7.5 cells. (A) Time course of production of HCVcc particles in cell-free supernatant of HCV-infected Huh7.5 cells. Huh7.5 cell monolayer at ∼ 70% confluency and containing ∼ 75% of HCV-infected cells was rinsed 3 times with a fresh medium and cultured at 37°C. Culture supernatant aliquots were collected at the indicated time points, filtered through 0.45-μm membrane filter, and stored at −80°C. HCV RNA was quantified by RT-PCR. In parallel, infectious titer of HCV was determined in Huh7.5 cells. (B-H) Inhibition of type I IFN production in pDCs by increasing quantity of HCVcc particles (MOI, left panels B,D,G) or sE2 glycoprotein (μg/mL, right panels C,E-F,H). pDCs exposed to HCVcc particles or sE2 for 1 hour were stimulated with TLR7/9 agonists. (B-C,G-H) CpG-A, influenza virus A/H3N2/Johannesburg (Flu, MOIFlu RNA = 100), HCV-infected Huh7.5 cells (Huh-HCV), or control Huh7.5 cells (Huh). (D-E) A total of 0.05, 0.1, or 5μM R848. Production of IFN-α and IFN-λ in cell-free supernatant was determined by ELISA 20 hours after stimulation. (F) Inhibition of IFN-α production is abrogated by treatment of E2 glycoprotein with 1 μg of anti-E2 mAb (AP33) at 37°C for 1 hour. MOI is expressed as a quantity of HCV RNA copies per pDC. Ctl indicates supernatant of CHO cells transfected with empty vector. Data are mean ± SEM of 3-6 independent experiments with pDCs from different donors. Some error bars are too small to be visible compared with the size of the symbol.
HCVcc particles and HCV E2 glycoprotein block production of IFN-α and IFN-λ induced by exposure of pDCs to HCV-infected Huh7.5 cells. (A) Time course of production of HCVcc particles in cell-free supernatant of HCV-infected Huh7.5 cells. Huh7.5 cell monolayer at ∼ 70% confluency and containing ∼ 75% of HCV-infected cells was rinsed 3 times with a fresh medium and cultured at 37°C. Culture supernatant aliquots were collected at the indicated time points, filtered through 0.45-μm membrane filter, and stored at −80°C. HCV RNA was quantified by RT-PCR. In parallel, infectious titer of HCV was determined in Huh7.5 cells. (B-H) Inhibition of type I IFN production in pDCs by increasing quantity of HCVcc particles (MOI, left panels B,D,G) or sE2 glycoprotein (μg/mL, right panels C,E-F,H). pDCs exposed to HCVcc particles or sE2 for 1 hour were stimulated with TLR7/9 agonists. (B-C,G-H) CpG-A, influenza virus A/H3N2/Johannesburg (Flu, MOIFlu RNA = 100), HCV-infected Huh7.5 cells (Huh-HCV), or control Huh7.5 cells (Huh). (D-E) A total of 0.05, 0.1, or 5μM R848. Production of IFN-α and IFN-λ in cell-free supernatant was determined by ELISA 20 hours after stimulation. (F) Inhibition of IFN-α production is abrogated by treatment of E2 glycoprotein with 1 μg of anti-E2 mAb (AP33) at 37°C for 1 hour. MOI is expressed as a quantity of HCV RNA copies per pDC. Ctl indicates supernatant of CHO cells transfected with empty vector. Data are mean ± SEM of 3-6 independent experiments with pDCs from different donors. Some error bars are too small to be visible compared with the size of the symbol.
Blockade of production of IFN-α in pDCs is mediated by HCV E2 glycoprotein
Despite our efforts to minimize the effect of bystander cellular inhibitory factors in viral stocks by their partial purification and the use of a low cytopathic HCV JFH-1 3M,18 it is possible that cellular material could participate, in addition to HCVcc particles, in the suppression of TLR7/9-mediated IFN-α production in pDCs. To address this issue, we assayed the specificity of HCVcc particle-induced inhibition of IFN-α production by the use of sE2 glycoprotein expressed in CHO cells (Figure 1C). As with HCVcc particles, we found that sE2 glycoprotein inhibited production of IFN-α in pDCs stimulated by coculture with HCV-infected Huh7.5 cells in a dose-dependent manner (P < .05 for [sE2] ≥ 0.91 μg/mL). sE2 glycoprotein inhibited production of IFN-α also in pDCs stimulated with CpG-A (Figure 1C; P < .05 for [sE2] ≥ 0.45 μg/mL) or suboptimal concentration of R848 (Figure 1E; P < .05 for [sE2] concentration ≥ 0.91 μg/mL). However, sE2 glycoprotein did not inhibit TLR7-mediated production of IFN-α stimulated by influenza virus (Figure 1C) or optimal concentration of R848 (Figure 1E).
To further show that inhibition of IFN-α was mediated by specific cellular binding of E2 glycoprotein, we exposed sE2 glycoprotein to anti-E2 antibody, AP3323 (Figure 1F). The CpG-A-induced production of IFN-α abrogated by sE2 glycoprotein was restored by preincubation of sE2 glycoprotein with anti-E2 mAb. Abrogation of the capacity to inhibit production of IFN-α by pDCs by anti-E2 mAb suggests that HCV E2 glycoprotein is involved in inhibition of IFN-α.
HCV E2 glycoprotein blocks TLR7/9-mediated production of IFN-λ in pDCs
To investigate whether, in addition to IFN-α, exposure of pDCs to HCV-infected hepatoma cells results in the production of IFN-λ and whether this production of IFN-λ is blocked by HCVcc particles and sE2 glycoprotein, we assayed selected supernatants previously tested for IFN-α (Figure 1B-C) for the presence of IFN-λ. Our results show that exposure of pDCs to HCV-infected Huh7.5 cells or CpG-A induced production of IFN-λ and that HCVcc particles and sE2 glycoprotein block this production (Figure 1G-H; P < .05 for MOI ≥ 10 and [sE2] ≥ 1.81 μg/mL).
HCVcc particles activate Akt and Erk1/2 kinases characteristic of signaling via regulatory receptors
To better understand the mechanism by which cell-free HCVcc particles and sE2 glycoprotein blocked production of IFN-α induced by cell-associated HCV, we compared the kinetics of signal transduction triggered in pDCs by different TLR7/9 and regulatory receptor agonists with those induced by both forms of HCV. The insufficient quantities of primary pDCs available for biochemical analyses led us to use dynamic phosphoflow cytometry in our studies. This technique permits the determination of signaling events at the single-cell level.26 First, we determined kinetics of phosphorylation of Akt and Erk1/2 in pDCs on exposure to control TLR7/9 agonists, R848 or CpG-A, or on crosslinking of regulatory receptors BDCA-2 or DCIR (Figure 2). TLR7/9 agonists R848 and CpG-A induced in pDCs phosphorylation of Akt, which gradually increased for 30 minutes and then remained stable. None of these assayed TLR7/9 agonists induced phosphorylation of Erk1/2. In contrast, triggering of regulatory receptors by crosslinking of BDCA-2 or DCIR induced rapid phosphorylation of Akt and Erk1/2 that peaked within 15 minutes of activation and then dropped down during the next 15 minutes. Then we determined kinetics of phophorylation induced by cell-associated and cell-free HCV; kinetics of phophorylation induced by HCV-infected and SGR-transfected Huh7.5 cells resembled that induced by TLR7/9 agonists, whereas kinetics of phophorylation induced by HCVcc particles resembled that induced by crosslinking of BDCA-2 or DCIR. Kinetics of phophorylation induced by mix of HCVcc particles with HCV-infected or SGR-transfected Huh7.5 cells resembled that induced by crosslinking of regulatory receptors. Taken together, these results show that (1) HCVcc particles trigger the same signaling pattern in pDCs as the crosslinking of regulatory receptors, (2) HCV-infected Huh7.5 cells induce in cocultured pDCs the same signaling pattern as TLR7/9 agonists, and (3) the effect of HCVcc particles dominates the effect of HCV-infected Huh7.5 cells.
Exposure of pDCs to HCVcc particles triggers the same activation signals as the crosslinking of DCIR and BDCA-2 regulatory receptors. Populations of magnetic bead-sorted pDCs were stimulated and followed by analysis of phosphorylation of Akt and Erk1/2 kinases by flow cytometry. Kinetics of phosphorylation of Akt and Erk1/2 in pDCs exposed to synthetic agonists CpG-A or R848, to HCV-infected Huh7.5 cells (Huh-HCV), or SGR-transfected Huh7.5 cells (Huh-SGR), to mock-infected Huh7.5 cells (Huh), to crosslinking with anti-DCIR or anti–BDCA-2 antibodies, to HCV particles (MOIHCV = 100), or to the mixtures of HCV particles with HCV-infected Huh7.5 cells (Huh-HCV + HCV particles). Data are mean ± SEM of 3-6 independent experiments with pDCs from different donors.
Exposure of pDCs to HCVcc particles triggers the same activation signals as the crosslinking of DCIR and BDCA-2 regulatory receptors. Populations of magnetic bead-sorted pDCs were stimulated and followed by analysis of phosphorylation of Akt and Erk1/2 kinases by flow cytometry. Kinetics of phosphorylation of Akt and Erk1/2 in pDCs exposed to synthetic agonists CpG-A or R848, to HCV-infected Huh7.5 cells (Huh-HCV), or SGR-transfected Huh7.5 cells (Huh-SGR), to mock-infected Huh7.5 cells (Huh), to crosslinking with anti-DCIR or anti–BDCA-2 antibodies, to HCV particles (MOIHCV = 100), or to the mixtures of HCV particles with HCV-infected Huh7.5 cells (Huh-HCV + HCV particles). Data are mean ± SEM of 3-6 independent experiments with pDCs from different donors.
HCVcc particles and HCV sE2 glycoprotein bind C-type lectin regulatory receptors BDCA-2 and DCIR
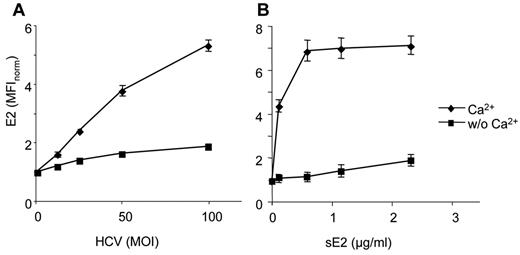
Because regulatory receptors of pDCs are members of the CLR or FcR superfamilies, we first assayed whether the binding of HCVcc particles and sE2 glycoprotein to pDCs is Ca2+-dependent (Figure 3). The binding of HCVcc particles (Figure 3A) or sE2 glycoprotein (Figure 3B) in the absence or presence of 0.9mM Ca2+ was determined by flow cytometry using Alexa-647–labeled anti-E2 mAb. Our results showed that both HCVcc particles and sE2 glycoprotein bind to pDCs by a Ca2+-dependent mechanism. Therefore, we investigated the possibility that sE2 glycoprotein binds to CLRs.
HCVcc particles and HCV E2 glycoprotein bind to pDCs in a Ca2+-dependent manner. Freshly isolated pDCs were exposed to HCVcc particles (A) or soluble E2 glycoprotein (B) in the presence or absence of Ca2+. HCVcc particles or E2 were detected with 5 μg/mL of anti-E2 mAb conjugated with Alexa-647. MOI is expressed as quantity of HCV RNA copies per pDC. Data are mean ± SEM of 3-6 independent experiments with pDCs from different donors.
HCVcc particles and HCV E2 glycoprotein bind to pDCs in a Ca2+-dependent manner. Freshly isolated pDCs were exposed to HCVcc particles (A) or soluble E2 glycoprotein (B) in the presence or absence of Ca2+. HCVcc particles or E2 were detected with 5 μg/mL of anti-E2 mAb conjugated with Alexa-647. MOI is expressed as quantity of HCV RNA copies per pDC. Data are mean ± SEM of 3-6 independent experiments with pDCs from different donors.
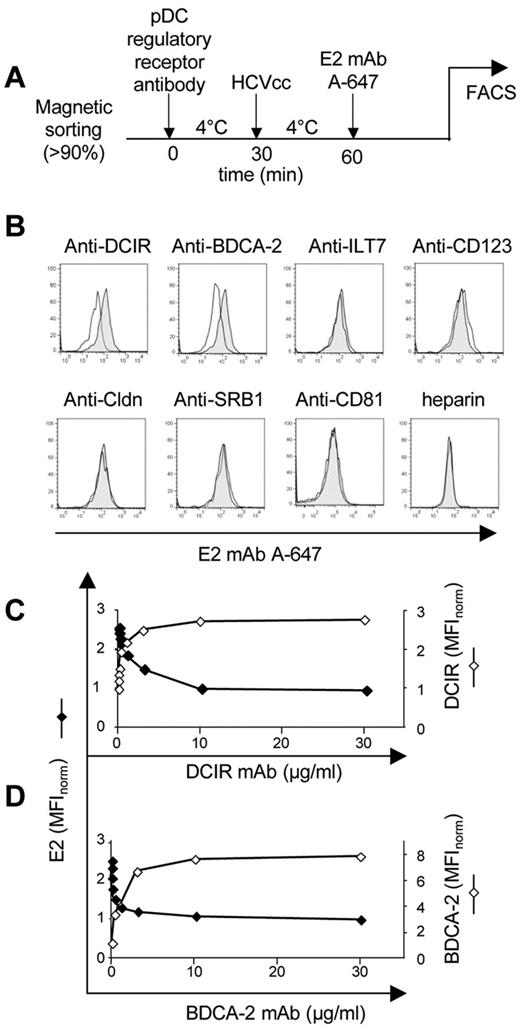
To identify HCV receptor(s) on pDCs, we blocked several molecules previously described as HCV attachment or entry factors as well as CLRs with saturating concentrations of specific antibodies before testing the binding of HCVcc particles (Figure 4A-B). In these experiments, we used antibodies against selected hepatocyte-entry receptors SR-BI, CD81, and claudin-121,22 as well as antibodies against CLRs BDCA-2 and DCIR, leukocyte-immunoglobulin-like receptor ILT7, and IL-3 receptor (CD123; negative control). Because heparan sulfates also bind HCV glycoproteins and were suggested to play a role in HCV capture from the bloodstream onto hepatocytes, we also assayed the effect of heparin on HCV binding to pDCs. Among tested antibodies, only anti-DCIR and anti–BDCA-2 reduced binding of HCVcc particles to pDCs.
Anti-DCIR and BDCA-2 mAbs block binding of HCVcc particles to pDCs. (A) Experimental flowchart. (B) pDCs were pretreated with antibodies against BDCA-2 and DCIR, ILT7, CD123, claudin-1, SR-BI, and CD81, and heparin for 30 minutes at 4°C (full line) or control isotype antibody or heparin control (shadow area). HCVcc particles (MOIHCV RNA = 100) were added to antibody-treated pDCs in the presence of Ca2+ at 4°C, and the binding to pDCs was determined 30 minutes later by anti-E2 mAb conjugated with Alexa-647 and analyzed by flow cytometry. (C-D) Saturating curves of anti-DCIR (C) and anti–BDCA-2 (D) mAbs in pDCs (right ordinate). Relative median fluorescent intensity (MFI) of anti-DCIR and anti–BDCA-2 was normalized to 1 at 0 μg/mL of respective antibodies. Inhibition of HCVcc binding by anti-DCIR (C) and anti–BDCA-2 (D; left ordinate). Representative result of one of 3 experiments is shown. Relative MFI of anti-E2 mAb was normalized to 1 at 30 μg/mL of anti-DCIR and anti–BDCA-2.
Anti-DCIR and BDCA-2 mAbs block binding of HCVcc particles to pDCs. (A) Experimental flowchart. (B) pDCs were pretreated with antibodies against BDCA-2 and DCIR, ILT7, CD123, claudin-1, SR-BI, and CD81, and heparin for 30 minutes at 4°C (full line) or control isotype antibody or heparin control (shadow area). HCVcc particles (MOIHCV RNA = 100) were added to antibody-treated pDCs in the presence of Ca2+ at 4°C, and the binding to pDCs was determined 30 minutes later by anti-E2 mAb conjugated with Alexa-647 and analyzed by flow cytometry. (C-D) Saturating curves of anti-DCIR (C) and anti–BDCA-2 (D) mAbs in pDCs (right ordinate). Relative median fluorescent intensity (MFI) of anti-DCIR and anti–BDCA-2 was normalized to 1 at 0 μg/mL of respective antibodies. Inhibition of HCVcc binding by anti-DCIR (C) and anti–BDCA-2 (D; left ordinate). Representative result of one of 3 experiments is shown. Relative MFI of anti-E2 mAb was normalized to 1 at 30 μg/mL of anti-DCIR and anti–BDCA-2.
We performed more precise analyses of HCVcc particles binding to the CLRs DCIR and BDCA-2 along with the saturation curves of mAbs against these regulatory receptors (Figure 4C-D). For both DCIR and BDCA-2, the binding of HCVcc particles to pDCs decreased with increasing concentration of antireceptor antibodies.
To determine directly whether HCVcc particles and sE2 glycoprotein bind DCIR and BDCA-2, we transiently transfected COS-7 cells with either DCIR or BDCA-2 expression vector or cotransfected with both expression vectors (Figure 5). After 48 hours, a high level (> 70%) of DCIR and BDCA-2 expression was achieved. We reproducibly observed HCVcc and HCV sE2 glycoprotein binding to the single-transfected cells expressing DCIR or BDCA-2, but not to mock-transfected cells. In addition, expression of both DCIR and BDCA-2 receptors on COS-7 cells resulted in an additive or weakly synergistic effect on HCVcc or sE2 binding compared with binding to COS-7 cells expressing each of the receptors alone (index of synergism IS ≥ 1.7).
HCVcc particles and HCV E2 glycoprotein bind BDCA-2 and DCIR-transfected or cotransfected COS-7 cells. (A-B) HCVcc particles (A) or E2 glycoprotein (B) binding to mock-transfected and BDCA-2- and DCIR-transfected or cotransfected COS-7 cells. The index of synergism (IS) is indicated at the MFI values showing synergistic effect on HCV/E2 binding to BDCA-2 and DCIR. It was determined from the following formula: the MFI of anti-E2 mAb after binding of HCVcc to COS-7 cells cotransfected with the combination of DCIR and BDCA-2 expression vectors divided by the sum of MFI of anti-E2 mAb after binding of HCVcc to COS-7 cells transfected separately with DCIR and BDCA-2 expression vectors; IS = MFIBDCA-2 + DCIR/(MFIBDCA-2 + MFIDCIR). The combinations resulting in the index of synergism > 1.5 were considered as synergistic. Data are mean ± SEM of 3 independent experiments.
HCVcc particles and HCV E2 glycoprotein bind BDCA-2 and DCIR-transfected or cotransfected COS-7 cells. (A-B) HCVcc particles (A) or E2 glycoprotein (B) binding to mock-transfected and BDCA-2- and DCIR-transfected or cotransfected COS-7 cells. The index of synergism (IS) is indicated at the MFI values showing synergistic effect on HCV/E2 binding to BDCA-2 and DCIR. It was determined from the following formula: the MFI of anti-E2 mAb after binding of HCVcc to COS-7 cells cotransfected with the combination of DCIR and BDCA-2 expression vectors divided by the sum of MFI of anti-E2 mAb after binding of HCVcc to COS-7 cells transfected separately with DCIR and BDCA-2 expression vectors; IS = MFIBDCA-2 + DCIR/(MFIBDCA-2 + MFIDCIR). The combinations resulting in the index of synergism > 1.5 were considered as synergistic. Data are mean ± SEM of 3 independent experiments.
HCV particles fail to inhibit production of type I and III IFNs in pDCs saturated with Fab fragments of BDCA-2 and DCIR mAbs
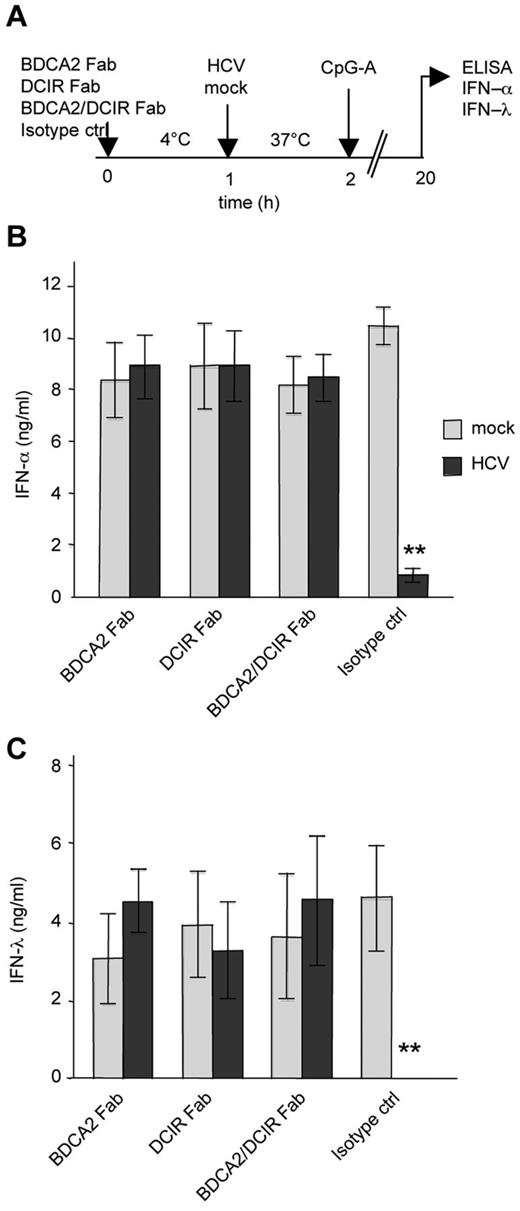
To demonstrate that blockade of the TLR7/9 agonist-induced production of IFN-α in pDCs is mediated by binding of HCVcc particles to regulatory receptors, we saturated DCIR and BDCA-2 with Fab fragments of specific antibodies before exposure to HCVcc and stimulation with CpG-A (Figure 6A). Saturation of DCIR, BDCA-2, or both receptors with Fab fragments of BDCA-2 and DCIR mAbs completely abolished the inhibition of IFN-α or of IFN-λ observed in isotype-treated pDCs exposed to HCV particles and stimulated with CpG-A (Figure 6B-C). In a control experiment in the absence of HCV particles, Fab fragments had no effect on type I and type III IFN production (Figure 6B-C). These results demonstrate that the interaction of E2 glycoprotein with BDCA-2 and DCIR triggers inhibition of TLR7/9-mediated production of IFN-α or IFN-λ in pDCs and that this inhibition can be blocked by Fab fragments of mAbs against BDCA-2 and DCIR.
Blocking of DCIR and BDCA-2 restores production of IFN-α and IFN-λ in pDCs exposed to HCVcc particles. (A) Experimental flowchart. pDCs were saturated with 20 μg/mL of Fab fragments of DCIR and/or BDCA-2 antibody at 4°C. Fab-saturated pDCs were then exposed to HCVcc particles (MOIHCV RNA = 100) and stimulated with CpG-A. (B-C) Production of IFN-α (B) or IFN-λ (C) in cell-free supernatant was determined by ELISA 20 hours after stimulation. Data are mean ± SEM of 3 independent experiments with pDCs from different donors. **P < .01.
Blocking of DCIR and BDCA-2 restores production of IFN-α and IFN-λ in pDCs exposed to HCVcc particles. (A) Experimental flowchart. pDCs were saturated with 20 μg/mL of Fab fragments of DCIR and/or BDCA-2 antibody at 4°C. Fab-saturated pDCs were then exposed to HCVcc particles (MOIHCV RNA = 100) and stimulated with CpG-A. (B-C) Production of IFN-α (B) or IFN-λ (C) in cell-free supernatant was determined by ELISA 20 hours after stimulation. Data are mean ± SEM of 3 independent experiments with pDCs from different donors. **P < .01.
Discussion
As the principal producers of type I and III IFNs, pDCs play a central role in antiviral immune responses.3-5 Although HCV particles induce in pDCs no or only weak production of type I IFN,11-15 transfer of HCV RNA from HCV-infected hepatocytes to cocultured pDCs is responsible for the production of large amounts of TLR7-mediated IFN-α.14 In the present study, we demonstrate that cell-free HCV particles or E2 glycoprotein antagonize the production of type I and III IFNs induced in pDCs by hepatoma cell-associated HCV. Thus, exposure of pDCs to cell-free or cell-associated form of the same virus results in a totally different outcome: activation or suppression of the principal pDC function, IFN production. These disparate effects are controlled by interaction of both viral forms with different types of pDC receptors. Although the cell-associated HCV triggers TLR7-dependent signaling,14 we determined that the cell-free HCV particles trigger signaling mediated by CLRs and that the CLR-triggered signaling induces inhibition of production of type I and III IFNs. HCV particles activate Akt and Erk1/2 kinases by the same kinetics as crosslinking of CLRs, which is reported to trigger in pDCs a B-cell receptor-like signaling pathway.7,8 Our recent observation that signaling induced by HCV particles or HCV-infected hepatoma cells does not result in activation of NF-κB pathway illustrates the multitude of mechanisms used by HCV to escape from immune recognition.16
We demonstrate, for the first time, that the HCV glycoprotein E2 is a natural ligand of BDCA-2 and DCIR and that, on functional level, blocking of BDCA-2 and DCIR with Fab fragments of specific antibodies protects pDCs against the inhibition of IFN production induced by HCV particles. The demonstration that Fab fragments of BDCA-2 and DCIR mAbs preserve the capacity of pDCs to produce type I and III IFNs in the presence of HCV particles and sensitize pDCs to TLR-mediated activation opens new horizons for the research on antiviral therapies based on TLR agonists. Both BDCA-2 and DCIR expressed in COS cells are able to bind HCV E2 glycoprotein separately. Selective blocking of each of the receptors was sufficient to suppress the inhibitory effect of the HCV particles, which suggests that both CLRs interact in HCV binding. This is also consistent with the synergistic effect of BDCA-2 and DCIR on E2 glycoprotein binding. Although recent reports show that the binding of HIV-1 glycoprotein gp120 and hepatitis B virus surface antigen with BDCA-2 is accompanied by a concomitant inhibition of TLR9-mediated production of IFN-α, direct effect of BDCA-2 interaction with HIV-1 or HBV particles on pDCs function was not yet demonstrated.24,27
The outcome of the antiviral response of pDCs depends on spatiotemporal characteristics of HCV-pDC interaction (ie, on the sequential order in which TLR7/9 or CLRs are engaged with the cell-free or the cell-associated HCV). When pDCs are exposed to the cell-free HCV before the cell-associated virus, HCV particles exert, via CLRs, a dominant negative effect on production of type I and III IFNs induced by cell-associated HCV. In the inverse order, the cell-associated HCV elicits via TLR7 a dominant positive effect on cell-free HCV particles, and IFNs are produced. This dominant positive effect of cell-associated HCV is probably at least partially facilitated by down-modulation of BDCA-2 and DCIR, as reported for TLR agonist-stimulated pDCs.9 pDCs with down-modulated BDCA-2 and DCIR cannot then sense HCV particles. Although the dominance of cell-free and cell-associated HCV could be studied in experimental settings, where pDCs can be exposed to HCV-infected cells in the absence of virions and vice versa, in infected liver pDCs are exposed simultaneously to both viral forms. Our results show that, if pDCs are exposed simultaneously to both viral forms, the dominance of HCV particles over the cell-associated virus prevails, as demonstrated by the phosphorylation pattern of Akt and Erk1/2 (Figure 2).
CLR-mediated inhibition of type I and III IFNs in pDCs exposed to HCV particles has a quantitative aspect. HCV particles efficiently block production of type I and III IFNs induced by cell-associated HCV, CpG-A, or suboptimal concentration of R848. In contrast, HCV particles fail to inhibit type I and III IFNs induced by optimal concentrations of R848 or influenza virus. This shows that HCV particles are able to inhibit some, but not all, TLR7/9 agonists, consistent with the observation that persons chronically infected with HCV are not immunocompromised.12 In our experimental setting, HCV particles elicited inhibitory effect on production of IFN-α induced by HCV-infected hepatoma cells at the MOI ≥ 10 HCV RNA-containing particles per pDC, corresponding to the virus load ≥ 107 HCV RNA copies/mL. Given that most chronically infected patients have levels of HCV RNA between 105 and 107 copies/mL of plasma, and that a higher quantity of HCV particles could be expected in infected liver, our experimental setting is relevant to conditions in infected liver.
In the present study, we demonstrate that, in addition to IFN-α, exposure of pDCs to HCV-infected hepatoma cells also induces production of IFN-λ and that binding of HCV particles or E2 glycoprotein to pDCs antagonized the production of both type I and III IFN. Genome-wide association studies have revealed important roles for the λ IFNs in the control of HCV infection and responses to antiviral therapy.28 Expression of IFN- λ is restricted mainly to pDCs and epithelial cells.6,29 Thus, the restricted expression pattern of the λ IFNs and their receptor confers tight control of IFN-λ responsiveness, in contrast to the situation with the ubiquitous type I IFN. Taking into account similarity of promoters of type I and III IFNs, induction of IFN-λ by exposure of pDCs to HCV-infected hepatoma cells is not surprising. Comparison of gene regulation of type I and III IFNs demonstrated dependence on NF-κB, IRF-3, and IRF-7 binding sites within the IFN-λ promoter.30 Thus, our demonstration that HCV-infected hepatoma cells do not stimulate phosphorylation of NF-κB can explain lower levels of type I and III IFNs induced by cell-associated HCV than those induced by synthetic agonists of TLR7/9.16
Activation of IFN-stimulated genes in infected liver, one of the hallmarks of HCV pathogenesis and chronic HCV infection, remains elusive with respect to the cellular source of type I and III IFNs. In infected liver, pDCs exposed to hepatocyte-associated HCV are good candidates for production of intrahepatic type I IFN,14,31,32 which not only activates expression of IFN-stimulated genes but also promotes greater activation of both pDCs and myeloid dendritic cells during virus infections.33 Suppression of pDC function by one form of the virus, whose other form activates this function, represents a novel mechanism of evasion of the immune system by viruses. Recent reports, which show that HIV-1–infected lymphocytes are more potent inducers of IFN-α than HIV-1 particles and that HIV-1 binds to BDCA-2 and DCIR, suggest that, in addition to HCV, a similar mechanism of immune evasion may be used by HIV-1.24,34-36 In view of the central role of pDCs in regulating the immune system, our results provide insight into the role of HCV-pDC interaction in HCV immune evasion and are important for our understanding of the mechanisms leading to the establishment of chronic HCV infection.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank T. Wakita for providing the HCV JFH-1 clone, C. M. Rice for the H/SG-neo (L + I) subgenomic replicon, and Genentech for the generous gift of AP33 antibody.
This work was supported by the French National Agency for AIDS Research and Viral Hepatitis (ANRS), Inserm, Institut Paoli Calmettes, and Plateform Cancer Immuno-Monitoring IBiSA, and by fellowships from ANRS (R.S.), Institut National du Cancer (INCa; G.F. and J.A.N.), the French Ministry of Higher Education and Research (J.F. and C.D.), the Algerian Ministry of Higher Education and Research, and Franco-Algerian Cooperation (B.A.).
The sponsors had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Authorship
Contribution: J.F. performed and designed research, analyzed data, and wrote the paper; B.A., C.D., F.G.-R., G.F., and C.T. performed research and analyzed data; V.S., T.F.B., J.A.N., and D.O. designed research and analyzed data; and I.H. and R.S. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ruzena Stranska, Inserm UMR1068, Centre de Recherche en Cancérologie de Marseille, BP30059, 27, boulevard Lei Roure, 13273 Marseille Cedex 09, France; e-mail ruzena.stranska@inserm.fr; and Ivan Hirsch, Inserm UMR1068, Centre de Recherche en Cancérologie de Marseille, BP30059, 27, boulevard Lei Roure, 13273 Marseille Cedex 09, France; e-mail: ivan.hirsch@inserm.fr.