The intricacies of cancer-platelet interactions are poorly understood. In this issue of Blood, Cho et al demonstrate that platelets enhance proliferation of ovarian cancer cells by a mechanism involving TGFβ.1
Thrombocytosis occurs in 10% to 50% of patients with solid malignancies. This has generally been labeled as “reactive” or “secondary,” implying that the tumors, even small, can stimulate platelet production. On the flipside, several studies have suggested that platelets modulate tumor growth and metastasis. The mechanisms for both of these phenomena have remained largely obscure.
The concept that platelets influence tumor progression is not new. In 1968, Gasic and colleagues first showed in animal models that induction of thrombocytopenia decreased tumor metastasis.2 Since then, several studies have strengthened evidence for these interactions and the role of platelets.3-6 Recently, Stone et al (including several investigators involved in the current report) found in a large cohort of 619 patients with epithelial ovarian cancers that patients with elevated platelet counts had poorer outcomes—advanced disease and shortened survival—than those with normal counts.7 Through a combination of multiple approaches, they delineated a paracrine signaling pathway by which ovarian tumors promote thrombocytosis. They proposed that tumor cells secrete IL-6, which stimulates hepatocyte thrombopoeitin production to promote thrombopoeisis. In support of this mechanism, they observed that in patients with ovarian cancer, platelet counts correlated with the levels of 2 factors known to stimulate platelet production, IL-6 and thrombopoeitin. In murine models, ovarian cancer cells produced IL-6, and inhibition of either IL-6 or thrombopoietin inhibited thrombocytosis. Of clinical importance, siltuximab, a monoclonal antibody that inhibits IL-6, also reduced platelet counts in patients with ovarian cancer.
Why might tumors promote platelet production? Stone et al provided compelling evidence that tumors use elevated platelet numbers to deliver signals that promote tumor growth. In murine ovarian cancer models, IL-6 neutralization not only blocked thrombocytosis, but also slowed tumor growth, as did thrombocytopenia induced by an antibody against GP1bα. In these models, platelets could be found outside of the tumor vasculature, and in vitro, could produce factors that increased proliferation of cultured ovarian cancer cells. However, the specific molecular signals provided by platelets were not defined.
It is in this continuum that the present study by Cho and colleagues becomes important by defining a potential player. The investigators show that platelets enhance the proliferation rate of human and murine ovarian cancer cells by a mechanism that does not require direct cell contact but is abrogated by fixation of platelets with formaldehyde.1 Blocking of adhesive interactions mediated by GPIbα, GPαIIbβ3, or P-selectin did not diminish the proliferative effect of platelets. However, a blocking antibody against TGFβ1, a protein released from platelets, reduced tumor cell proliferation. Down-regulation of the cognate receptor TGFBR1 on tumor cells showed a similar effect. These findings are supported by the in vivo demonstration that weekly platelet transfusions into mice with orthotopic ovarian tumors enhanced the proliferation index in the tumors. In line with this, the authors observe that ovarian tumors from patients with thrombocytosis had an increased proliferative index compared with those with normal counts.
These studies come on the heels of an elegant study reported last year by Labelle et al, who provided convincing evidence for a direct cross-talk between platelets and colon carcinoma and breast carcinoma cells.5 They showed that platelet-derived TGFβ and direct platelet-tumor cell contact activated both TGFβ/Smad and NF-kβ pathways and, importantly, induced transition of tumor cells to an invasive mesenchymal-like phenotype and enhanced tumor metastasis in vivo.
The sum total of these and other studies is that platelets contribute in a major way to the growth and dissemination of malignancies. The molecular events are now becoming clearer. These insights reveal new opportunities to modulate specific mechanisms, including platelet number, function, and interaction with tumors, to achieve therapeutic benefits in patients with ovarian and other cancers (see figure). In a murine tumor model, a combination of the chemotherapeutic agent paclitaxel with the humanized IL-6–blocking antibody siltuximab reduced ovarian tumor growth over that with either alone, and this was associated with a lowering of platelet counts by siltuximab.7
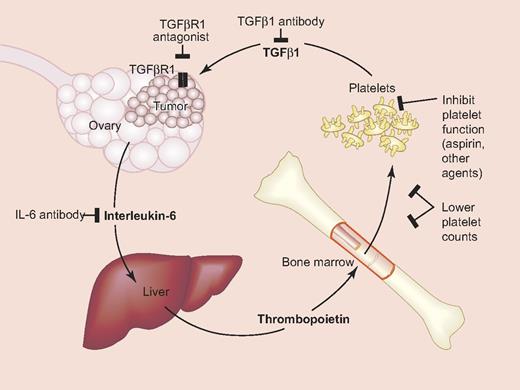
Platelet-ovarian cancer signaling pathways and potential therapeutic targets. Interleukin-6 secreted by ovarian cancer cells stimulates hepatic thrombopoietin (TPO) production, which drives thrombopoiesis in the bone marrow and thrombocytosis. TGFβ secreted from platelets interacts with cancer cells to increase proliferation. Also shown are potential targets for modulating these interactions. Modified from Stone at al.7 Professional illustration by Paulette Dennis.
Platelet-ovarian cancer signaling pathways and potential therapeutic targets. Interleukin-6 secreted by ovarian cancer cells stimulates hepatic thrombopoietin (TPO) production, which drives thrombopoiesis in the bone marrow and thrombocytosis. TGFβ secreted from platelets interacts with cancer cells to increase proliferation. Also shown are potential targets for modulating these interactions. Modified from Stone at al.7 Professional illustration by Paulette Dennis.
Also relevant to this discussion is the role of the effects of aspirin in modulating the behavior of tumors, which has recently received substantial attention.8-10 In a pooled analysis of 46 randomized trials of daily aspirin use of any treatment duration for the primary or secondary prevention of vascular disease, Rothwell and colleagues showed that irrespective of dose, cancer deaths were reduced by 15%.9 The benefit was seen within 3 years for high doses (> 300 mg/day) and after 5 years for low doses (< 300 mg/day). In a companion study they reported a lower risk of developing metastasis in individuals on aspirin.10 Although these studies have limitations, the evidence overall is compelling that aspirin seems to reduce cancer incidence and death across different subgroups and cancer sites; the strongest evidence has been in colorectal tumors. Interestingly, Cho et al also found that aspirin partially reduced the proliferative effect of platelets in vitro on ovarian cancer cells.1
What emerges from the amalgamation of these disparate pieces of the puzzle is a strong rationale for targeting platelet-tumor cross-talk for therapeutic gain. The modalities appear feasible and in sight—and the field is ripe for such approaches. It is time to reign in the platelet-tumor cross-talk.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal