Abstract
Light transmission aggregometry (LTA) is used worldwide for the investigation of heritable platelet function disorders (PFDs), but interpretation of results is complicated by the feedback effects of ADP and thromboxane A2 (TxA2) and by the overlap with the response of healthy volunteers. Over 5 years, we have performed lumi-aggregometry on 9 platelet agonists in 111 unrelated research participants with suspected PFDs and in 70 healthy volunteers. Abnormal LTA or ATP secretion test results were identified in 58% of participants. In 84% of these, the patterns of response were consistent with defects in Gi receptor signaling, the TxA2 pathway, and dense granule secretion. Participants with defects in signaling to Gq-coupled receptor agonists and to collagen were also identified. Targeted genotyping identified 3 participants with function-disrupting mutations in the P2Y12 ADP and TxA2 receptors. The results of the present study illustrate that detailed phenotypic analysis using LTA and ATP secretion is a powerful tool for the diagnosis of PFDs. Our data also enable subdivision at the level of platelet-signaling pathways and in some cases to individual receptors. We further demonstrate that most PFDs can be reliably diagnosed using a streamlined panel of key platelet agonists and specified concentrations suitable for testing in most clinical diagnostic laboratories.
Introduction
An adequate number of normally functioning platelets is essential in arresting hemorrhage from an injured blood vessel. Subjects with heritable platelet function disorders (PFDs) experience lifelong abnormal bleeding, typically at mucocutaneous sites or elsewhere after trauma or invasive procedures. This group of disorders is highly heterogeneous and can arise through defects in surface membrane receptors, signaling pathways, granule formation and secretion, cytoskeletal remodeling, and expression of procoagulant activity.1,2 Diagnosis of the severe PFDs, such as Glanzmann thrombasthenia and Bernhard-Soulier syndrome, or syndromic PFDs, such as Hermansky Pudlak syndrome, is usually straightforward because of the characteristic clinical and laboratory features.3-6 Conversely, poor standardization of platelet function tests and the lack of consensus surrounding minimal diagnostic criteria have hampered diagnosis of the remaining PFDs.
Light transmission aggregometry (LTA) is a long-established method for the diagnosis of PFDs. Responses to individual test agonists are typically classified as “abnormal” if numerical parameters such as the level of maximal aggregation fall outside of the reference range of healthy donor controls or if there are qualitative differences between test and control aggregation curves.7,8 However, most PFDs are associated with “abnormal responses” to multiple agonists and it is unclear whether it is the number of abnormal responses8 or the specific patterns of abnormal responses9 that is more powerful in the diagnosis of PFDs. The additional diagnostic value of ATP secretion in the diagnosis of PFDs is not known.
We have previously defined reference intervals for LTA and dense granule secretion to 3 concentrations of 9 platelet agonists in a cohort of 20 healthy volunteers.9 We now present data from the United Kingdom Genotyping and Phenotyping of Platelets (GAPP) study,10 which includes extended data on 70 healthy donor controls that validate our previous reference intervals.9 We also describe LTA and ATP secretion test results in a prospectively studied cohort of 111 patients with suspected PFDs registered at United Kingdom Hemophilia Comprehensive Care Centres. Detailed analysis of LTA and ATP secretion using several concentrations of the extended panel of 9 agonists confirmed PFDs in approximately 60% of participants. More than 80% of this group exhibited defects in Gi receptor signaling, the thromboxane A2 (TxA2) pathway, or dense granule secretion. Further, a retrospective blinded analysis revealed that approximately 90% of PFDs can be identified with a restricted range of concentrations of 6 platelet agonists, which we term a “streamlined agonist panel.” This indicates that reliable diagnosis of PFDs and definition of the major pathways is feasible in nonspecialist clinical diagnostic laboratories using a combination of LTA and ATP secretion.
Methods
Selection of participants
Participants with bleeding symptoms.
Participants with suspected platelet function defects were referred from August 2006 to September 2011 from United Kingdom Comprehensive Care Hemophilia Centres and were invited to participate in this study if they satisfied all of the following inclusion criteria: (1) abnormal bleeding symptoms compatible with PFD (spontaneous mucocutaneous bleeding or abnormal bleeding at other sites following trauma or invasive procedures); (2) results from coagulation factor tests all within local laboratory reference intervals (minimum panel of prothrombin time, activated thromboplastin time, Clauss fibrinogen activity, VWF ristocetin cofactor activity, and activities of factors VIII, IX and XI; and (3) absence of demonstrable for acquired platelet dysfunction. Patients with existing diagnoses of Glanzmann thrombasthenia, Bernard-Soulier syndrome, or Hermansky-Pudlak syndrome and those with platelet counts < 100 or > 450 × 109/L were excluded. Laboratory testing was deferred in participants exposed within 2 weeks to drugs known to affect platelet function. Information on the age and sex of the participants is given in the “Results.”
Healthy controls.
Healthy donor volunteers 18 years of age or older were also included in this study. Controls were considered healthy if they did not have a history of bleeding symptoms, did not require long-term medical therapy, and had refrained from drugs known to influence platelet function in the previous 2 weeks.
Ethics.
This study was approved by the National Research Ethics Service Committee West Midlands–Edgbaston (REC reference: 06/MRE07/36), and participants and controls gave written informed consent in accordance with the Declaration of Helsinki.
Platelet agonists and other reagents
ADP, adrenaline, and U46619 were purchased from Sigma-Aldrich. Arachidonic acid (sodium salt) was from Cayman Chemical. Horm collagen was from Nycomed Austria. The PAR-1 receptor–specific peptide SFLLRN was from Alta Bioscience Laboratory (University of Birmingham, Birmingham, United Kingdom), and the PAR-4 receptor–specific peptide AYPGKF and collagen-related peptide (CRP) were from Dr Richard Farndale (Cambridge University, Cambridge, United Kingdom). Luciferin-luciferase reagent (Chrono-Lume) was from the Chrono-log Corporation. AR-C67085 was a gift from AstraZeneca. Rhodocytin was a gift from Dr Johannes Eble (Frankfurt University Hospital, Frankfurt, Germany).
Platelet preparation and measurement of aggregation and secretion
Blood from participants and a simultaneous healthy volunteers was taken into sodium citrate (3.8%) and transported at ambient temperature (approximately 20°C) to the laboratory. In the majority of cases, blood was taken in the adjacent clinic, but in some cases, samples from participants and healthy volunteers were transported over distances of up to 100 miles by courier to Birmingham. We have demonstrated previously that the results in platelet-rich plasma (PRP) from samples that have been transported in this way are indistinguishable from those from locally collected samples provided that the PRP is prepared at the site of testing.9
PRP was prepared by centrifugation at 200g for 10 minutes in a spinout rotor. Platelet aggregation and ATP secretion were measured in PRP using a dual Chrono-log lumi-aggregometer (model 460 VS). Autologous platelet-poor plasma was used to set the aggregation scale before each study according to the manufacturer's instructions. All experimentation was performed within 6 hours of preparation of the PRP, as described previously.9 Priority was given to time-sensitive agonists such as ADP and adrenaline, which were always used first and within 4 hours of blood collection.9,11 No adjustment was made to the platelet count because there was no significant difference in the aggregation curves to platelet agonists within the normal range of platelet count as shown by comparison of the response with 3 platelet agonists in healthy volunteers at the 2 extreme ends of the normal range (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). This is consistent with previous results.12-15
Platelets from the participants and healthy volunteers were routinely exposed to ADP (3, 10, 30, and 100μM), adrenaline (10, 30, and 100μM), collagen (0.3, 1, and 3 μg/mL), CRP (1, 3, and 10 μg/mL), PAR-1 (10, 30, and 100μM), and PAR-4 (100, 250, and 500μM) peptides, arachidonic acid (0.5, 1, and 1.5mM), U46619 (1 and 3mM), and ristocetin (1, 1.25, 1.5, and 2 mg/mL). The responses to ADP and arachidonic acid were the first to be investigated because these are the major 2 feedback agonists and a significant number of participants were found to have defects in Gi signaling or in the TxA2 pathway.16 This information was used to guide subsequent testing, notably in cases where the platelet volume was limiting.
P2RY12 analysis
The P2RY12 coding sequence was amplified from genomic DNA and sequenced directly, as described previously.17
Data analysis
Aggregation responses at each agonist concentration were classified as abnormal by reference to a bank of local healthy volunteers.9 ATP secretion was calculated by the addition of a known concentration of ATP (4nM) and normalized to a platelet count of 1 × 108 platelets/mL (supplemental Figure 2). Results are shown as means ± SD. Statistical analysis was performed using a 1-tailed Student t test and Cohen κ statistics.
Results
The 111 index cases were composed of 81 female and 30 male patients. There were 70 healthy volunteers. The mean age and SD of the participants with a clinically diagnosed PFD was 41 ± 16 years and 31 ± 19 years for female and male participants, respectively. A full distribution of the ages of participants is shown in supplemental Figure 3.
LTA and ATP secretion were determined for 9 platelet agonists (up to 3 concentrations) on each participant alongside a control (see “Methods”). The response of each participant was evaluated by a minimum of 2 experts in platelet function testing and compared with the control on the day and to a reference range that originally consisted of 20 controls, but which had built up to 70 controls by the end of the study. A retrospective analysis was performed to verify the original diagnoses and a small number of adjustments were made. This approach revealed that 64 of 111 (58%) participants (43 female and 21 male) had a clear defect in platelet aggregation or ATP secretion to several concentrations of platelet agonists.
Major subgroups of PFDs
Comparing the pattern of response in the 64 participants with an identified defect in LTA or ATP secretion revealed that 54 of 64 (84%) could be assigned to 3 major diagnostic groups: those with defects in (1) Gi signaling, (2) the TxA2 pathway, and (3) granule secretion. The distribution of defects is in shown in Table 1 and the characteristic features of each subgroup are described in the following sections.
Classification of participants with mild platelet-based bleeding defects
| Type of platelet defect . | No. of participants . | % of participants . |
|---|---|---|
| Membrane Gi signaling | 21 | 32.8% |
| TxA2 pathway | 14 | 21.9% |
| GPVI | 4 | 6.2% |
| Gq | 1 | 1.6% |
| Dense granule | 19 | 29.7% |
| Complex | 5 | 7.8% |
| Total | 64 | 100% |
| Type of platelet defect . | No. of participants . | % of participants . |
|---|---|---|
| Membrane Gi signaling | 21 | 32.8% |
| TxA2 pathway | 14 | 21.9% |
| GPVI | 4 | 6.2% |
| Gq | 1 | 1.6% |
| Dense granule | 19 | 29.7% |
| Complex | 5 | 7.8% |
| Total | 64 | 100% |
Participants who exhibited a defect in platelet function were subdivided on the basis of their major platelet phenotype, as described in the text.
Gi-like defect.
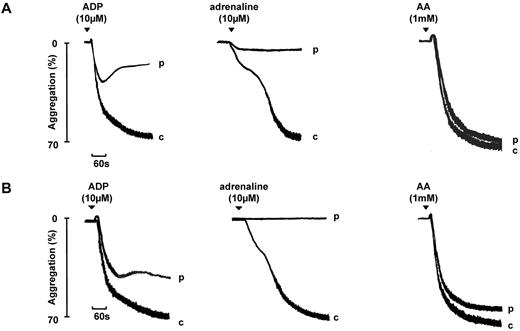
A total of 21 (32.8%) of the 64 index cases were diagnosed with a Gi-like defect. This subgroup exhibited a defect in aggregation and secretion to the 2 Gi-coupled heterotrimeric receptors agonists ADP and adrenaline. A key diagnostic feature was a transient aggregation to ADP (10μM), a reduced or absent primary wave with no secondary wave of aggregation to adrenaline, and the absence of ATP secretion to both agonists. Similar numbers of participants had a reduced primary wave or absent primary response to adrenaline.16 In comparison, high concentrations of ADP and adrenaline induced sustained aggregation in the 70 healthy volunteers. Representative traces to ADP and adrenaline are shown in Figure 1.
Aggregation to ADP and adrenaline in 2 participants diagnosed with a Gi-like defect. Aggregation in 2 participants (p) diagnosed with a Gi-like defect is shown. The participant in panel A shows a partial primary wave response to adrenaline, whereas for a second participant shown in panel B, the primary wave is absent. “c” indicates the control (healthy volunteer). Note that the biphasic aggregation to ADP shown in panel B would eventually decline. The patterns of aggregation are representative of other participants diagnosed with a Gi-like defect.
Aggregation to ADP and adrenaline in 2 participants diagnosed with a Gi-like defect. Aggregation in 2 participants (p) diagnosed with a Gi-like defect is shown. The participant in panel A shows a partial primary wave response to adrenaline, whereas for a second participant shown in panel B, the primary wave is absent. “c” indicates the control (healthy volunteer). Note that the biphasic aggregation to ADP shown in panel B would eventually decline. The patterns of aggregation are representative of other participants diagnosed with a Gi-like defect.
Reduced aggregation and secretion to low and intermediate concentrations of other platelet agonists, most notably collagen, was also observed in this group, which is consistent with impairment of the known feedback role of ADP. At higher agonist concentrations, sustained aggregation and a normal level of ATP secretion was usually seen. A robust response to 1mM arachidonic acid (Figure 1) helps to distinguish this group of participants from those with a defect in the TxA2 pathway, as described in the next section.
TxA2 pathway defect.
A total of 14 (21.9%) of the 64 index cases were diagnosed with a TxA2 pathway defect. This was characterized by a marked and selective defect in aggregation and secretion to arachidonic acid (1mM) as illustrated by the representative trace in Figure 2. In 3 of the 14 participants with this defect, a reduced response to the TxA2-mimetic U46619 was also seen, indicating a defect at the level of the TxA2 receptor or its downstream signaling cascade. A normal aggregation response to U46619 is evidence for a defect in conversion of arachidonic acid to TxA2 because the response to U46619 is insensitive to cyclooxygenase blockade.9,18 Aggregation and secretion to low concentrations of other platelet agonists was also reduced in this group, which is consistent with the positive feedback role of TxA2. For most agonists, full recovery was seen at higher concentrations (not shown), with the exceptions of ADP and adrenaline which exhibited a slowly decaying aggregation response and severely reduced or absent secondary wave, respectively (Figure 2).
Aggregation in a participant diagnosed with a TxA2 pathway defect. Aggregation in a participant (p) diagnosed with a TxA2 pathway defect. The TxA2 pathway defect also results in the abolition of response to arachidonic acid (1mM) and impairment in response to other agonists, including ADP and adrenaline, but not to U46619, indicating a defect in arachidonic acid metabolism. “c” indicates control (healthy volunteer). The pattern of aggregation is representative of other participants diagnosed with a defect in arachidonic metabolism.
Aggregation in a participant diagnosed with a TxA2 pathway defect. Aggregation in a participant (p) diagnosed with a TxA2 pathway defect. The TxA2 pathway defect also results in the abolition of response to arachidonic acid (1mM) and impairment in response to other agonists, including ADP and adrenaline, but not to U46619, indicating a defect in arachidonic acid metabolism. “c” indicates control (healthy volunteer). The pattern of aggregation is representative of other participants diagnosed with a defect in arachidonic metabolism.
Dense granule–secretion defect.
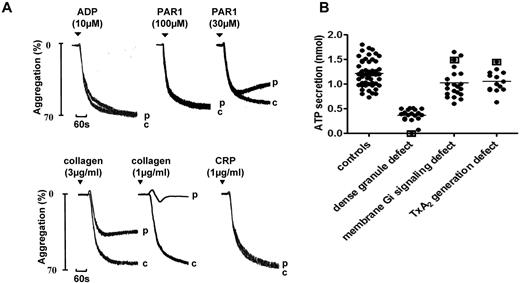
A total of 19 (30%) of the 64 index cases were diagnosed with a dense granule secretion defect that could be due to a defect in either storage or secretion. The level of secretion was dependent on the platelet count, which was normalized to the observed response to PAR-1 (Figure 3A) or PAR-4 peptides (see “Methods”). Participants were considered to have a significant defect in secretion when the response fell outside of the normal range determined in controls. A decrease in ATP secretion was observed in response to all agonists, even though several, including PAR-1, PAR-4, and CRP, induced maximal, sustained aggregation at high concentrations. However, there was a reduction in aggregation to low concentrations of most platelet agonists, which is consistent with impairment of the feedback role of ADP and TxA2. The response to a low concentration of collagen was markedly impaired, as illustrated in the representative trace in Figure 3A. One agonist that showed a minimal change in its dose-response curves for aggregation was ADP, which is consistent with our previous conclusion that secretion plays a minimal role in aggregation to the nucleotide.9
Aggregation and ATP secretion in a participant diagnosed with a dense granule defect. (A) Aggregation in a participant diagnosed with a defect in dense granule secretion on the basis of a significantly reduced level of secretion to high concentrations of PAR-1–specific peptide and other platelet agonists, including PAR-4–specific peptide and CRP relative to a panel of controls. “c” indicates control (healthy volunteer). The pattern of aggregation is representative of other participants diagnosed with a secretion disorder. (B) ATP secretion was measured alongside aggregation in a Born lumi-aggregometer in PRP using Chrono-Lume reagent for the detection of ATP. The degree of ATP secretion (after normalization to platelet count, supplemental Figure 2) to PAR-1–specific peptide (100μM) in healthy volunteers and participants diagnosed with defective dense granule secretion is shown. Participants identified with mutations in the P2Y12 (present study), TxA2 receptors,10 and HPS-819 are identified by square brackets.
Aggregation and ATP secretion in a participant diagnosed with a dense granule defect. (A) Aggregation in a participant diagnosed with a defect in dense granule secretion on the basis of a significantly reduced level of secretion to high concentrations of PAR-1–specific peptide and other platelet agonists, including PAR-4–specific peptide and CRP relative to a panel of controls. “c” indicates control (healthy volunteer). The pattern of aggregation is representative of other participants diagnosed with a secretion disorder. (B) ATP secretion was measured alongside aggregation in a Born lumi-aggregometer in PRP using Chrono-Lume reagent for the detection of ATP. The degree of ATP secretion (after normalization to platelet count, supplemental Figure 2) to PAR-1–specific peptide (100μM) in healthy volunteers and participants diagnosed with defective dense granule secretion is shown. Participants identified with mutations in the P2Y12 (present study), TxA2 receptors,10 and HPS-819 are identified by square brackets.
The PAR-1 peptide (100μM) elicited a limited degree of secretion of ATP in all index cases diagnosed with a secretion disorder (Figure 3B), with the exception of the index case (and also other related family members) with an HPS-8 mutation (homozygous).19 Secretion to the PAR-1 peptide (100μM) in participants diagnosed with a Gi or TxA2 pathway defect, as well as one participant with a homozygous P2Y12 receptor mutation (see below) and 2 with a heterozygote TxA2 receptor defect (see below and refer to Mumford et al18 ) fell within the normal range (Figure 3B). These results demonstrate that the majority of participants diagnosed with a secretion disorder elicit a limited degree of dense granule secretion and that the maximal level of secretion to a high concentration of a PAR-1 peptide is unaffected by mutations in the P2Y12 ADP or TxA2 receptors.
Other, less common subgroups of PFDs
Comparison of LTA and ATP secretion results in the remaining 10 of 64 (16%) participants, along with additional tests, revealed several further subgroups shown in Table 1, namely defects in the GPVI signaling pathway, Gq receptor signaling, and in the ADP and TxA2 receptors (confirmed by gene sequencing). In 2 other participants, the pattern of defects could not be assigned to any of these groups and is described as complex to indicate the presence of defects in multiple pathways. These subgroups are described in the following sections.
P2Y12 ADP receptor mutation.
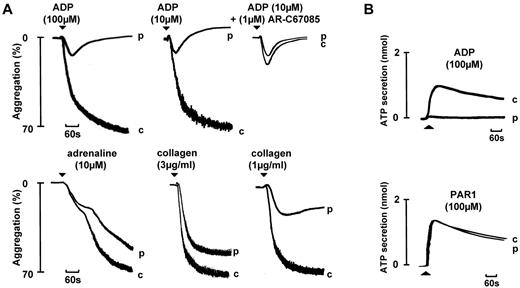
The index case was a 31-year-old Asian woman with a lifelong history of severe bruising and prolonged bleeding from cuts, who had required a blood transfusion following a cesarean section. Her parents were first cousins, although neither had a history of bleeding. Her brother also had a lifelong history of prolonged bleeding from cuts but was not available for investigation. Her full blood count and basal coagulation tests were within the normal range. The participant's platelets exhibited a weak, transient aggregation and absence of secretion in response to a high concentration of ADP (100μM) that was preceded by shape change (Figure 4A). The response was not altered in the presence of the P2Y12 receptor antagonist AR-C67085, whereas the addition of the antagonist reduced the response to ADP in the corresponding healthy volunteer (Figure 4A). The participant's platelets exhibited a biphasic aggregation to adrenaline and a mild defect in aggregation to low concentrations of other platelet agonists, most notably collagen (consistent with the feedback role of ADP in supporting activation), which normalized at higher concentrations (Figure 4A). The level of ATP secretion by maximal concentrations of the PAR-1 peptide was within the normal range, indicating that the participant did not have a secretion defect (Figure 4B). ADP had no effect on cAMP formation by PGE1, whereas adrenaline induced a marked decrease in the second messenger (supplemental Figure 4).
Aggregation and secretion in a participant with a homozygous P2Y12 mutation that prevents receptor expression. Aggregation and secretion in a participant (p) with a homozygous mutation in P2Y12 that introduces a frame-shift mutation early in the coding sequence (c.36delG, p.Gly12fs). Responses are shown alongside a control (c). The PRP platelet count in the control and participant were 4.1 × 108/mL and 3.9 × 108/mL, respectively.
Aggregation and secretion in a participant with a homozygous P2Y12 mutation that prevents receptor expression. Aggregation and secretion in a participant (p) with a homozygous mutation in P2Y12 that introduces a frame-shift mutation early in the coding sequence (c.36delG, p.Gly12fs). Responses are shown alongside a control (c). The PRP platelet count in the control and participant were 4.1 × 108/mL and 3.9 × 108/mL, respectively.
This profile is indicative of a defect in the P2Y12 ADP receptor. In confirmation of this, genomic DNA sequencing revealed that the participant was homozygous for a single base deletion at nucleotide position 36 of the P2RY12 gene. This mutation is predicted to cause a frameshift leading to introduction of a premature stop codon (c.36delG, p.Gly12fs), resulting in a failure to express the P2Y12 receptor.
TxA2 receptor mutations.
We have recently described a participant with a function-disrupting heterozygous mutation in the TxA2 receptor.18 The defining features of this participant were a marked defect in aggregation and secretion to low concentrations of arachidonic acid and U46619. We have since identified a second participant who is heterozygous for a distinct mutation in the TxA2 receptor, which alters receptor trafficking (Nisar and Mundell, unpublished data, January 2012). In both participants, the defect in aggregation and secretion is similar to that in participants with defective arachidonic acid metabolism described above, with the significant difference being the reduction in response to U46619.
GPVI pathway defect.
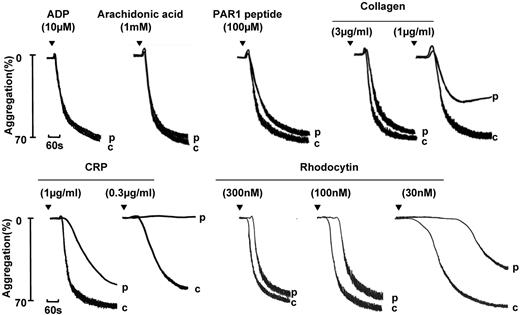
We have identified 4 index patients with a GPVI-like defect on the basis of a selective reduction in aggregation and secretion to the GPVI-specific agonist CRP, whereas the response to ADP, arachidonic acid, and the PAR-1– and PAR-4–specific peptides were within the normal range (Figure 5 and data not shown). Aggregation and secretion to low concentrations of collagen were also reduced, although recovery was seen at higher concentrations, as illustrated by the representative traces in Figure 5. The weaker effect on collagen relative to CRP can be accounted for by the presence of a second collagen receptor on the platelet surface, integrin α2β1, and by the marked dependency of the response of collagen on the feedback actions of ADP and TxA2. The snake venom toxin rhodocytin activates platelets through the C-type lectin receptor CLEC-2, which signals through a signaling cascade this is closely related to that of GPVI.20 The demonstration of an impairment in aggregation to rhodocytin, as illustrated in Figure 5, is indicative of a signaling defect downstream of GPVI or CLEC-2.
Aggregation and secretion in a participant with a GPVI-like defect. Aggregation in a participant (p) diagnosed with a GPVI-like defect on the basis of a reduced response to CRP and to rhodocytin. A similar pattern of aggregation was observed in other participants diagnosed with a GPVI-like defect. “c” indicates control.
Aggregation and secretion in a participant with a GPVI-like defect. Aggregation in a participant (p) diagnosed with a GPVI-like defect on the basis of a reduced response to CRP and to rhodocytin. A similar pattern of aggregation was observed in other participants diagnosed with a GPVI-like defect. “c” indicates control.
Gq-like defect.
One male index case and his sister from a nonconsanguineous relationship were observed to have a partial defect in aggregation and secretion to intermediate concentrations of agonists that signal through the heterotrimeric G proteins Gq and G13, namely PAR-1– and PAR-4–specific peptides and the TxA2 analog U46619 (supplemental Figure 5). In contrast, the responses to ADP and CRP were only marginally inhibited, arguing against a general defect in platelet activation. This is further supported by the similar nature of the response to the phorbol ester PMA in the participant's platelets (supplemental Figure 5). Therefore, the defect is putatively at the level of Gq or G13 or their downstream signaling proteins.
Streamlined agonist panel testing
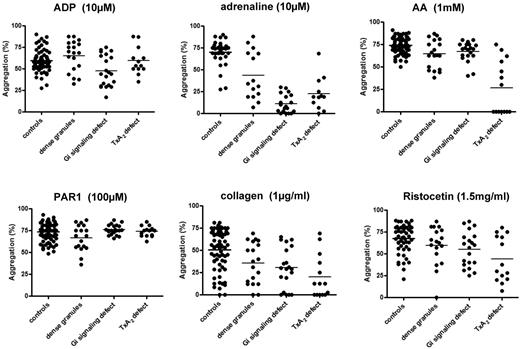
We next considered whether a reduced panel of LTA and ATP secretion tests would be sufficient to enable reliable diagnosis of PFD. After review of the above results, we developed a streamlined panel of agonists and concentrations based on their ability to diagnose and discriminate the above subgroups of PFDs (Table 2). We then undertook a comparison of the diagnosis of a PFD using this streamlined panel with the original agonist panel. These studies took into account the extent and time course of aggregation (noting whether it was transient or delayed) and the level of secretion of ATP, because the extent of maximal aggregation alone does not distinguish between the major 3 platelet function defect groups from controls (Figure 6).
Streamlined panel of agonists with interpretative notes for diagnosing platelet function defects
| Agonist . | Concentration . | % of maximal aggregation, mean ± SD . | Expected . | Abnormal pattern and further testing . |
|---|---|---|---|---|
| ADP* | 10μM | 59.8% ± 12.5% | Maximal, sustained aggregation and secretion | Reduced or transient aggregation and absent secretion: use 30μM |
| Adrenaline* | 10μM | 70.3% ± 13.7% | Biphasic aggregation with secretion coincident with second phase | Reduced or absent primary wave and absent secretion: use 30μM |
| Arachidonic acid | 1mM | 71.0% ± 8.5% | Maximal, sustained aggregation and secretion | Absent or delayed/reduced aggregation and secretion: use U46619 (3μM) |
| PAR-1 receptor–specific peptide (SFLLRN) | 100μM | 73.8% ± 11.1% | Maximal, sustained aggregation and marked secretion | Transient aggregation and reduced secretion: use PAR-4 receptor–specific peptide (AYPGKF; 500μM). |
| Collagen* | 1 μg/mL | 50.4% ± 22.5% | Sustained aggregation and secretion | Reversible aggregation and absent secretion: use 3 μg/mL and collagen-related peptide (CRP; 3 μg/mL) or convulxin |
| Ristocetin | 1.5 mg/mL | 70.8% ± 16.1% | Maximal sustained aggregation (often biphasic) and secretion | Reduced or absent aggregation and secretion |
| Agonist . | Concentration . | % of maximal aggregation, mean ± SD . | Expected . | Abnormal pattern and further testing . |
|---|---|---|---|---|
| ADP* | 10μM | 59.8% ± 12.5% | Maximal, sustained aggregation and secretion | Reduced or transient aggregation and absent secretion: use 30μM |
| Adrenaline* | 10μM | 70.3% ± 13.7% | Biphasic aggregation with secretion coincident with second phase | Reduced or absent primary wave and absent secretion: use 30μM |
| Arachidonic acid | 1mM | 71.0% ± 8.5% | Maximal, sustained aggregation and secretion | Absent or delayed/reduced aggregation and secretion: use U46619 (3μM) |
| PAR-1 receptor–specific peptide (SFLLRN) | 100μM | 73.8% ± 11.1% | Maximal, sustained aggregation and marked secretion | Transient aggregation and reduced secretion: use PAR-4 receptor–specific peptide (AYPGKF; 500μM). |
| Collagen* | 1 μg/mL | 50.4% ± 22.5% | Sustained aggregation and secretion | Reversible aggregation and absent secretion: use 3 μg/mL and collagen-related peptide (CRP; 3 μg/mL) or convulxin |
| Ristocetin | 1.5 mg/mL | 70.8% ± 16.1% | Maximal sustained aggregation (often biphasic) and secretion | Reduced or absent aggregation and secretion |
Different concentrations of agonists with the percentage of maximal aggregation ± SD are shown.
ATP secretion from dense granules should be measured for the following agonist concentrations: ADP (30μM), adrenaline (30μM), arachidonic acid (1mM), PAR-1–specific peptide (100μM), and collagen (3 μg/mL).
Maximal aggregation response in participants with platelet function defects and healthy volunteers. The percentage of maximal aggregation was measured in a Born lumi-aggregometer in PRP in response to the shown concentrations of the following agonists: ADP, adrenaline, arachidonic acid, collagen, PAR-1–specific peptide, and ristocetin. The results are shown as the percentage increase in light transmission relative to platelet-poor plasma.
Maximal aggregation response in participants with platelet function defects and healthy volunteers. The percentage of maximal aggregation was measured in a Born lumi-aggregometer in PRP in response to the shown concentrations of the following agonists: ADP, adrenaline, arachidonic acid, collagen, PAR-1–specific peptide, and ristocetin. The results are shown as the percentage increase in light transmission relative to platelet-poor plasma.
Two independent experts were blinded to previous diagnoses and reviewed data that would have been created using the streamlined agonist panel in 94 cases that were suitable for full analysis. Interobserver variation was minimal, with an agreement of approximately 90% (κ statistic 0.829, P < .001). A mutually acceptable consensus was subsequently reached for the 10% of patients with a difference of opinion. The diagnoses from this process were then compared with the previous historical diagnoses using the expanded agonist list (Table 3). There was a significant level of agreement with a κ statistic of 0.721 (P < .001). In addition, the sensitivity (87%), specificity (86%), negative predictive value (84%), and positive predictive value (88%) provided further verification of the streamlined agonist panel (Table 3).
Comparison of the expanded agonist panel and a streamlined agonist panel in diagnosing platelet function defect
| . | Expanded agonist panel positive . | Expanded agonist panel negative . | . |
|---|---|---|---|
| Streamlined agonist panel positive | 45 | 6 | Positive predictive value 88% |
| Streamlined agonist panel negative | 7 | 36 | Negative predictive value 84% |
| Sensitivity ( 87% ) | Specificity ( 86% ) |
| . | Expanded agonist panel positive . | Expanded agonist panel negative . | . |
|---|---|---|---|
| Streamlined agonist panel positive | 45 | 6 | Positive predictive value 88% |
| Streamlined agonist panel negative | 7 | 36 | Negative predictive value 84% |
| Sensitivity ( 87% ) | Specificity ( 86% ) |
Table shows a comparison between diagnoses of platelet function defect using the expanded agonist panel and a streamlined agonist panel. The sensitivity (87%), specificity (86%), negative predictive value (84%), and positive predictive value (88%) are shown. The kappa statistic was 0.721 (P < .001).
Discussion
The most widely used test for platelet function is LTA, which monitors the increase in light transmission through a suspension of platelets as aggregation proceeds. Among the advantages of this test are its relative simplicity, the ability to monitor responses to individual agonists over time, and (in our experience) its reproducibility at the level of each donor. Furthermore, it can be combined with real-time monitoring of ATP secretion in a lumi-aggregometer by the addition of the luciferin-luciferase reagent. The drawbacks of this method include the time taken to perform the assays and the fact that many investigators consider it to be operator dependent and to give inconsistent results. The interpretation of aggregation traces is also complex because of the feedback effects of redundant platelet activation pathways, although this applies to all tests of platelet function. Several of these limitations can be minimized by standardization of the aggregation and secretion assays, as performed in this study. A similar standardization of LTA was also used in a recent study of 229 participants to detect participants with platelet dysfunction.8
Defining the platelet phenotype through the analysis of aggregation and secretion to 9 platelet agonists, alongside other functional tests, provides important information on the defective pathway(s), and in some cases the defective protein, allowing targeted genetic analysis. It is not practical for hospital laboratories to follow this procedure because of the time taken for the analysis and the relatively low numbers of participants that are referred to each center. Nevertheless, a limited analysis of platelet aggregation using 1 or 2 concentrations of the “standard” platelet agonists (ADP, adrenaline, arachidonic acid, collagen, and ristocetin) is extremely valuable in aiding diagnosis.8
In the present study, we have validated the use of a streamlined panel of 6 platelet agonists (Table 3) by comparing the diagnoses made with the extended agonist panel. This streamlined agonist panel could be used in nearly all clinical testing centers and would serve to guide further subtyping of the PFD by a specialist laboratory or a clinical research study through functional investigations and targeted genotyping or analysis of whole-exome sequencing data. The functional tests could include an expanded range of platelet agonists and more specialized tests, such as measurement of second messengers (cAMP and Ca2+ elevation), TxA2 formation, shear-based assays of platelet adhesion and aggregation, and flow cytometric measurements of α-granule secretion and glycoprotein receptor levels. The streamlined agonist list would also identify nearly all of the 40% of patients in whom such tests would appear to have limited value.
The majority of participants with an identified defect in platelet function (approximately 80%) were assigned to 1 of 3 groups characterized by defects in Gi signaling, TxA2 formation, and dense granule secretion, which emphasizes the importance of these 3 pathways in supporting platelet activation during hemostasis. In the majority of these cases, the causative gene mutation(s) is not known and the value of the subgrouping is to enable further functional studies and targeted gene sequencing/gene interrogation.
The relatively small number of participants with defects in the Gq pathway is perhaps surprising given the importance of this pathway in platelet activation. However, it may reflect the fact that several platelet receptors signal through Gq-mediated pathways, namely the PAR-1, PAR-4, P2Y12, and TxA2 receptors, thereby reducing the impact of a partial defect. Alternatively, a mutation in this pathway could cause a more severe phenotype in other tissues that could be lethal in utero. The small number of participants with defects in the GPVI pathway is consistent with the relatively mild nature of bleeding associated with defects in the collagen receptor.21
There are several possible explanations for the failure to identify a defect in platelet function in almost 40% of participants suspected of having a platelet disorder. These include the likely possibility that they do not have a defect in platelet activation pathways, but rather an abnormality in another component of the hemostatic response, such as increased fibrinolysis or an impairment of vascular integrity. Alternatively, it is possible that they have a defect that has not been detected in the platelet function tests that have been performed, such as an enhanced activity of an inhibitory pathway. The limited repertoire of platelet function tests used in the present study may also have contributed to the failure to diagnose a platelet defect. However, in work that is not reported in this study, we have performed additional platelet tests in many of the participants who have been investigated in this study, including measurement of platelet aggregation on collagen at intermediate rate of shear (1000/s), and investigation of the inhibitory action of prostacyclin and sodium nitroprusside, which elevate cAMP and cGMP, respectively, and clot retraction. No defect was observed in any of these responses in patients for whom aggregation and secretion fell within the normal range.
The phenotyping of platelets has been used to direct genotyping and has led to the identification of participants with a homozygous defect in the P2Y12 ADP receptor gene (present study) and 2 heterozygous mutations in the TxA2 receptor, one of which has been described previously.18 The homozygous P2Y12 ADP receptor gene mutation reported in the present study is the 10th mutation to be reported in the nucleotide receptor.10 A third heterozygous mutation in the TxA2 receptor has also been reported recently.22 The only other reported mutation in the TxA2 receptor, Arg60Leu,23 may be a rare single nucleotide polymorphism because there is no clear defect in function of the receptor in cell line studies.24,25 We have not identified participants with mutations in the PAR-1 and PAR-4 thrombin receptors or the collagen receptor GPVI. There are no reported participants with mutations in the PAR-1 and PAR-4 receptors, possibly because they are incompatible with life, and only 2 patients with mutations in GPVI have been reported.26,27 The small number of participants with mutations in platelet surface receptors suggests that receptor mutations account form a relatively small proportion of mutations that give rise to bleeding symptoms, with the majority being in intracellular signaling pathways or functional processes such as secretion or TxA2 formation. Identifying the mutations that give rise to these function disorders requires a more high-throughput approach such as that offered by whole-exome sequencing or a targeted second-generation sequencing array.28
The present study represents a comprehensive investigation of participants with a clinically suspected platelet disorder alongside healthy volunteers. The participants have been grouped on the basis of the identified disorder, with the majority having defects in one of the major feedback pathways of platelet activation: Gi signaling, the TxA2 pathway, and dense granule secretion. A few of these participants have defects at the level of the ADP and TxA2 receptor, but the majority have mutations downstream of receptor activation, necessitating the use of second-generation sequencing and targeted interrogation based on the functional results to establish the causative mutation(s). Based on the present results, we developed a streamlined platelet agonist list that could be performed in most clinical testing centers and a sequence of interpretation (Table 3). This interpretation takes into account the pattern, but not the magnitude, of the defect in aggregation and whether secretion is also defective.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Milan Fernando and Kevin Horner for technical support and Paul Carter for helping with data analysis.
This work was supported by the British Heart Foundation (RG/09/007/27917 and PG/10/36/02) and the Wellcome Trust (093994 to G.L.). S.P.W. holds a British Heart Foundation chair (CH/03/003). M.L. is supported by the Canadian Institute of Health Research and the British Heart Foundation (PG/11/31/28835).
Wellcome Trust
Authorship
Contribution: B.B.D. designed and performed the research, collected, analyzed, and interpreted the data, and wrote the first draft of the manuscript; G.C.L. and M.L. have led the research governance of the GAPP study since October 2010, revised the manuscript, and contributed to the analysis of the results; D.B. performed the specialized platelet test; M.E.D. analyzed the P2RY12 gene and revised the manuscript; M.M. and A.M. recruited participants and revised the manuscript; J.T.W. recruited participants, interpreted the data, and contributed to and revised the manuscript; and S.P.W. designed the study, interpreted the data, and revised the draft and final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Ban B. Dawood, Centre for Cardiovascular Sciences, College of Medical and Dental Sciences, University of Birmingham, Wolfson Drive, Edgbaston, Birmingham B15 2TT, United Kingdom; e-mail: b.b.dawood@bham.ac.uk.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal