Abstract
The adaptive immune system can be a potent defense mechanism against cancer; however, it is often hampered by immune suppressive mechanisms in the tumor microenvironment. Coinhibitory molecules expressed by tumor cells, immune cells, and stromal cells in the tumor milieu can dominantly attenuate T-cell responses against cancer cells. Today, a variety of coinhibitory molecules, including cytotoxic T lymphocyte–associated antigen-4, programmed death-1, B and T lymphocyte attenuator, LAG3, T-cell immunoglobulin and mucin domain 3, and CD200 receptor, have been implicated in immune escape of cancer cells. Sustained signaling via these coinhibitory molecules results in functional exhaustion of T cells, during which the ability to proliferate, secrete cytokines, and mediate lysis of tumor cells is sequentially lost. In this review, we discuss the influence of coinhibitory pathways in suppressing autologous and allogeneic T cell–mediated immunity against hematologic malignancies. In addition, promising preclinical and clinical data of immunotherapeutic approaches interfering with negative cosignaling, either as monotherapy or in conjunction with vaccination strategies, are reviewed. Numerous studies indicate that coinhibitory signaling hampers the clinical benefit of current immunotherapies. Therefore, manipulation of coinhibitory networks is an attractive adjuvant immunotherapeutic intervention for hematologic cancers after standard treatment with chemotherapy and hematopoietic stem cell transplantation.
Introduction
Despite the powerful aspects of immune reactions, most often tumor cells are able to evade immune recognition and destruction. Mechanisms exploited by tumor cells to escape T cell–mediated immunity include disruption of antigen presentation, down-regulation of HLA molecules, secretion of immune suppressive cytokines, as well as recruitment of regulatory T cells (TREG) and myeloid-derived suppressor cells.1 In the last decade, another powerful immune suppressive mechanism gained much attention: the repressive action of coinhibitory molecules.2 Activation of T cells is predominantly dependent on both costimulatory and coinhibitory members, including members of the B7/ CD28 family. The balance between positive and negative cosignals determines the functionality of T cells during immunity and tolerance. In addition to the native role of cosignaling, tumor cells can evade immune control by down-regulating costimulatory molecules, such as CD80 and CD86, and up-regulating various coinhibitory ligands, thereby limiting the therapeutic potential of current immunotherapy against cancer.
Standard treatment for hematologic cancers includes chemotherapy and radiotherapy, which reduce tumor burden and can induce long-term remission. Moreover, in the past years, new therapeutics, including imatinib, dasatinib, rituximab, bortezomib, and lenalidomide, have been developed that target tumor cells. However, drug resistance and relapse remain major problems. In addition, cellular immunotherapy is an attractive treatment option to cure hematologic malignancies. Such cell-based immunotherapies include allogeneic stem cell transplantation (alloSCT), T-cell and NK-cell adoptive transfer, and vaccination-based approaches using various antigen formulations or dendritic cells (DCs). AlloSCT can be regarded as the most powerful cell-based immunotherapy, because of the graft-versus-tumor (GVT) responses constituted by alloreactive T cells.3 These alloreactive T-cell responses eradicate the malignant cells on recognition of polymorphic HLA-presented peptides, known as minor histocompatibility antigens (MiHA). AlloSCT greatly enhanced the cure rate for aggressive hematologic cancers, although many patients fail to launch productive immune responses and develop relapses. Moreover, a major drawback of alloSCT is the occurrence of GVHD, a potentially life-threatening side effect predominantly caused by alloreactive T cells recognizing healthy tissues, notably the skin, liver, and gastrointestinal tract. Because hemato-restricted MiHA are solely expressed by the redundant patient hematopoietic system and the hematologic malignancy, they hold the key to separate GVT from GVHD.4 Studies by us and others demonstrated that the cellular immunotherapies described earlier in the “Introduction” are often hampered by the action of coinhibitory molecules that attenuate tumor-reactive T-cell responses, resulting in suboptimal clinical results. This review will address the role of coinhibitory molecules in immune evasion by hematologic malignancies and discuss options to circumvent T-cell inhibition without severe adverse effects. In addition, we address whether a differential effect of coinhibitory molecules exists in GVT and GVHD, creating an opportunity to limit GVHD toxicity without dampening antitumor immunity.
Coinhibitory molecules in hematologic malignancies
Today a variety of coinhibitory molecules have been implicated in immune escape of cancer. Here, we discuss the coinhibitory molecules involved in suppressing antitumor immunity against hematologic malignancies (summarized in Table 1).
Major coinhibitory molecules and their corresponding binding partners involved in attenuating antitumor immunity
| Receptor . | Binding partners . | |||
|---|---|---|---|---|
| Name . | Expression pattern . | Name . | Expression on normal cells . | Expression on malignant cells . |
| CTLA-4 | Activated T, TREG | CD80/CD86 | T, B, DCs, macrophages | Down-regulated on AML, MM18,20 |
| PD-1 | Activated T and B, NKT, monocytes, myeloid cells | PD-L1 | Activated T, B, DCs, macrophages, monocytes, nonlymphoid tissues | AML, NHL, MM20,32 |
| PD-L2 | DCs, monocytes | AML, NHL, MM20,32 | ||
| BTLA | T, B, DCs, myeloid cells | HVEM | T, B, DCs, NK, myeloid cells and nonlymphoid tissues | AML, CLL, NHL, MM67,68,75 |
| LAG-3 | Activated T, TREG, B, pDCs, NK | MHC-II | Activated T, B, DCs, macrophages, monocytes, endothelium | Down-regulated in tumors1 |
| TIM-3 | Th1 CD4+ T, CD8+ T, DCs, NK, monocytes, epithelium | Galectin-9 | CD4 T cells, Treg, DCs, fibroblasts, granulocytes, endothelium | AML, lymphoma93,94 |
| CD200R | Activated T, B, NK, DCs, mast cells, myeloid cells, neutrophils | CD200 | Activated T, B, DCs, thymocytes, endothelium, nonlymphoid tissues | AML, CLL, MM84,85,108 |
| Receptor . | Binding partners . | |||
|---|---|---|---|---|
| Name . | Expression pattern . | Name . | Expression on normal cells . | Expression on malignant cells . |
| CTLA-4 | Activated T, TREG | CD80/CD86 | T, B, DCs, macrophages | Down-regulated on AML, MM18,20 |
| PD-1 | Activated T and B, NKT, monocytes, myeloid cells | PD-L1 | Activated T, B, DCs, macrophages, monocytes, nonlymphoid tissues | AML, NHL, MM20,32 |
| PD-L2 | DCs, monocytes | AML, NHL, MM20,32 | ||
| BTLA | T, B, DCs, myeloid cells | HVEM | T, B, DCs, NK, myeloid cells and nonlymphoid tissues | AML, CLL, NHL, MM67,68,75 |
| LAG-3 | Activated T, TREG, B, pDCs, NK | MHC-II | Activated T, B, DCs, macrophages, monocytes, endothelium | Down-regulated in tumors1 |
| TIM-3 | Th1 CD4+ T, CD8+ T, DCs, NK, monocytes, epithelium | Galectin-9 | CD4 T cells, Treg, DCs, fibroblasts, granulocytes, endothelium | AML, lymphoma93,94 |
| CD200R | Activated T, B, NK, DCs, mast cells, myeloid cells, neutrophils | CD200 | Activated T, B, DCs, thymocytes, endothelium, nonlymphoid tissues | AML, CLL, MM84,85,108 |
T indicates T cells; B, B cells; pDCs, plasmacytoid dendritic cells; NK(T), natural killer (T) cells; CLL, chronic lymphoid leukemia; and (B-)NHL, (B-cell) non-Hodgkin lymphoma.
CTLA-4
Expression and function of CTLA-4.
Cytotoxic T lymphocyte associated antigen-4 (CTLA-4; CD152) was the first coinhibitory molecule identified and is partly similar to the cosignaling molecule CD28.5 However, whereas CD28 is constitutively expressed on the membrane of naive T cells, CTLA-4 is primarily localized in intracellular compartments and rapidly translocates to the cell membrane on T-cell activation. The inhibitory function of CTLA-4 was revealed in knockout mice, which developed lethal lymphoproliferative disease with multiorgan T-cell infiltration.6 Like CD28, CTLA-4 has an extracellular domain containing the MYPPPY binding motif, enabling both receptors to interact with CD80 (B7-1) and CD86 (B7-2) expressed by antigen-presenting cells (APCs). However, the binding affinity of CTLA-4 for these ligands is 10- to 100-fold higher, thereby outcompeting CD28 and promoting immune inhibition.7
As CTLA-4 is up-regulated on TCR ligation, it plays an important role in dampening effector T-cell activation and regulating immune homeostasis. In addition, CTLA-4 signaling in immunosuppressive TREG mediates the control of autoreactive T cells, as in vivo interference with CTLA-4 on these cells elicited pathologic autoimmunity.8 The effect of CTLA-4 interference could be the result of depletion and/or inhibition of TREG. Wing et al showed that TREG-specific CTLA-4 deficiency resulted in impaired suppressive TREG function because CTLA-4 enables the down-regulation of CD80/CD86 on APCs,9 which can be partly the result of endocytosis of CD80 and CD86 by TREG.10 This renders a less stimulatory APC, resulting in a lasting cell-extrinsic inhibitory effect. CTLA-4 signaling can attenuate adaptive immune responses in chronic viral infections and cancer. CTLA-4 as such is not a marker of exhausted cells, but elevated levels on viral antigen-specific T cells correlated with their dysfunction in patients with chronic viral infections, which could be restored by CTLA-4 blockade.11 In addition, in cancer, high expression of CTLA-4 was correlated to antigen-specific T cell dysfunction in metastatic melanoma.12 In various CD80 and CD86-positive solid tumor models, monotherapy with CTLA-4 blocking antibody resulted in elimination of established tumors and long-lasting antitumor immunity.13 Several clinical trials have been performed with anti–CTLA-4 antibodies, mostly with ipilimumab in melanoma. Interestingly, an increase in overall survival of melanoma patients has been observed.14 However, not all patients gain clinical benefit, and individual responses are hard to predict. Furthermore, the occurrence of adverse toxic effects remains a problem. Interestingly, in one trial, patients responding to ipilimumab were reported to have high titers of anti-MICA antibodies, probably because of enhanced CD4+ T-cell function resulting in increased antibody responses. These antibodies may revert the functional inhibition of NK and CD8+ T cells induced by tumor-secreted MICA. In 2011, the FDA and European Medicines Agency approved ipilimumab treatment for advanced melanoma, paving the way for further exploration of therapies targeting coinhibitory molecules in cancer.15 Although anti–CTLA-4 treatment works in vivo, either alone or in combination with vaccines, in vitro CTLA-4 blockade has not been very successful in reversing T-cell dysfunction. This might be the result of limitations of the in vitro models, as CTLA-4 blockade probably exerts its in vivo action via multiple immune mediators (eg, effector T cells, TREG, antibody responses).16
CTLA-4 in hematologic malignancies.
Numerous experimental and clinical studies have demonstrated that coinhibitory molecules hamper T-cell immunity against hematologic cancers in both the autologous and allogeneic settings (Tables 2 and 3). For instance, a causal relationship between CTLA-4 and TREG was demonstrated in lymphoma patients.17 A large proportion of the lymphoma-infiltrating lymphocytes was identified as CTLA-4+ TREG, and TREG-mediated T-cell suppression could be abrogated by CTLA-4 blockade. In addition, CTLA-4:CD80/86 interactions also take place between T cells and tumor cells. In multiple myeloma (MM) patients, CD86 but not CD80 was expressed by tumor cells, whereas CTLA-4 was up-regulated on T cells, resulting in anergy of tumor-specific T cells.18 In concordance with these results, T cells from chronic lymphocytic leukemia patients responded to anti-CD3 activation by a decrease in CD28 and an increase in CTLA-4 expression, resulting in an inhibitory phenotype.19 Similar to MM, we and others showed that acute myeloid leukemia (AML) cells heterogeneously express CD86, but CD80 levels are generally low or absent.20,21 As CD80 and CD86 can mediate either T-cell stimulation via CD28 or T-cell inhibition via CTLA-4, their role in the induction of tumor-specific T-cell immunity was investigated in an AML model.22 Expression of CD86 on AML resulted in tumor rejection, whereas CD80+ AML tumors grew progressively. The latter observation was shown to be CTLA-4 dependent, as blockade with anti–CTLA-4 resulted in clearance of CD80+ AML cells.
Outcome of interference with murine coinhibitory molecules
| Molecule . | Therapy . | Tumor . | Outcome . | Reference . |
|---|---|---|---|---|
| Autologous | ||||
| CTLA-4 | Anti–CTLA-4 + ova-DC vaccination | Thymoma | Improved tumor rejection, enhanced antigen-specific T-cell responses | 23 |
| CTLA-4 | Anti–CTLA-4; CTLA-4 deletional knockout | AML | Increased survival and improved tumor rejection | 22 |
| CTLA-4 | Anti–CTLA-4 | AML | Enhanced T-cell response, prolonged survival | 54 |
| PD-1 | Anti–PD-L1; PD-1 knockout | MM | Delayed tumor growth; complete tumor rejection in PD-1 knockout | 49 |
| PD-1 | HSCT + whole cell vaccination + anti–PD-L1 | MM | Increased survival | 52 |
| PD-1 | Anti–PD-L1; PD-1 knockout | AML | Enhanced T-cell response, improved tumor rejection, increased survival | 50 |
| PD-1/TIM-3 | Anti–PD-L1 and/or mTim-3 hFc | AML | Delayed tumor growth on monotherapy, improved tumor rejection on combined blockade | 93 |
| CD200 | Anti-CD200 | AML | Increased survival | 86 |
| CD200 | Anti-CD200 | B-CLL | Improved tumor rejection | 87 |
| CD200 | Anti-CD200 | B-cell lymphoma | Delayed tumor growth | 88 |
| Allogeneic | ||||
| CTLA-4 | Anti–CTLA-4 | AML | Enhanced T-cell response and GVHD early after BMT; enhanced tumor-specific T-cell response later after BMT with low GVHD | 28 |
| PD-1 | Anti–PD-L1 | Lymphoma | Enhanced T-cell response | 63 |
| PD-1 | Anti–PD-L1 | None | Enhanced alloreactive T-cell response, no GVHD | 64 |
| PD-1 | Anti–PD-L1; PD-1 knockout | CML | Increased survival | 65 |
| BTLA | BTLA agonist | B-cell lymphoma | Early after BMT: prevention of GVHD; later after BMT: no effect on GVHD; effective GVT response | 78 |
| BTLA | BTLA agonist | Mastocytoma/T-cell lymphoma | Inhibition of alloreactive T-cell response, prevention from GVHD | 79 |
| HVEM | Specific blockade of BTLA binding | None | Prevention from acute GVHD | 80 |
| PD-1H/ VISTA | PD-1H/VISTA agonist | None | Prevention from acute GVHD | 103 |
| Molecule . | Therapy . | Tumor . | Outcome . | Reference . |
|---|---|---|---|---|
| Autologous | ||||
| CTLA-4 | Anti–CTLA-4 + ova-DC vaccination | Thymoma | Improved tumor rejection, enhanced antigen-specific T-cell responses | 23 |
| CTLA-4 | Anti–CTLA-4; CTLA-4 deletional knockout | AML | Increased survival and improved tumor rejection | 22 |
| CTLA-4 | Anti–CTLA-4 | AML | Enhanced T-cell response, prolonged survival | 54 |
| PD-1 | Anti–PD-L1; PD-1 knockout | MM | Delayed tumor growth; complete tumor rejection in PD-1 knockout | 49 |
| PD-1 | HSCT + whole cell vaccination + anti–PD-L1 | MM | Increased survival | 52 |
| PD-1 | Anti–PD-L1; PD-1 knockout | AML | Enhanced T-cell response, improved tumor rejection, increased survival | 50 |
| PD-1/TIM-3 | Anti–PD-L1 and/or mTim-3 hFc | AML | Delayed tumor growth on monotherapy, improved tumor rejection on combined blockade | 93 |
| CD200 | Anti-CD200 | AML | Increased survival | 86 |
| CD200 | Anti-CD200 | B-CLL | Improved tumor rejection | 87 |
| CD200 | Anti-CD200 | B-cell lymphoma | Delayed tumor growth | 88 |
| Allogeneic | ||||
| CTLA-4 | Anti–CTLA-4 | AML | Enhanced T-cell response and GVHD early after BMT; enhanced tumor-specific T-cell response later after BMT with low GVHD | 28 |
| PD-1 | Anti–PD-L1 | Lymphoma | Enhanced T-cell response | 63 |
| PD-1 | Anti–PD-L1 | None | Enhanced alloreactive T-cell response, no GVHD | 64 |
| PD-1 | Anti–PD-L1; PD-1 knockout | CML | Increased survival | 65 |
| BTLA | BTLA agonist | B-cell lymphoma | Early after BMT: prevention of GVHD; later after BMT: no effect on GVHD; effective GVT response | 78 |
| BTLA | BTLA agonist | Mastocytoma/T-cell lymphoma | Inhibition of alloreactive T-cell response, prevention from GVHD | 79 |
| HVEM | Specific blockade of BTLA binding | None | Prevention from acute GVHD | 80 |
| PD-1H/ VISTA | PD-1H/VISTA agonist | None | Prevention from acute GVHD | 103 |
B-CLL indicates B-cell chronic lymphoid leukemia; and BMT, bone marrow transplantation.
Outcome of interference with human coinhibitory molecules
| Molecule . | Therapy . | Tumor . | Outcome . | Reference . |
|---|---|---|---|---|
| CTLA-4 | Ipilimumab, in vivo | NHL | Two of 4 tumor regression, no increase in vaccine-specific T-cell responses, reduction in TREG number early after treatment; toxicity: mainly grade 1 or 2, 1 times grade 3 | 24 |
| CTLA-4 | Ipilimumab, in vivo | Relapsed/refractory B-cell NHL | Two of 18 clinical response, 5 of 16 enhanced T-cell response to recall Ag; toxicity: mainly grade 1 or 2, 6 of 18 grade 3 | 25 |
| CTLA-4 | Ipilimumab after alloSCT, in vivo | AML, CML, CLL, HL, NHL, MM | Three of 29 clinical response; toxicity: no induction of GVHD, 4 of 29 organ-specific immune adverse events | 29 |
| CTLA-4 | Anti–CTLA-4, ex vivo | HL | Abrogated TREG suppression | 17 |
| CTLA-4 | Anti–CTLA-4, ex vivo | CLL | Enhanced tumor-specific T-cell response | 109 |
| PD-1 | BMS-936,558, ex vivo | ALL, AML, CML, NHL, MM | Enhanced alloreactive T-cell response | 20 |
| PD-1 | Anti–PD-L1, ex vivo | NHL | Enhanced T-cell response | 52 |
| PD-1 | Anti–PD-L1 and anti–PD-L2, ex vivo | HL | Restored T-cell response | 53 |
| PD-1 | Anti–PD-L1, ex vivo | HCV lymphoma | Abrogated TREG suppression, reduction in Treg number | 55 |
| PD-1 | CT-011, in vivo | AML, CLL, HL, NHL, MDS, MM | Six of 17 clinical response, 1 complete remission, no toxicity | 57 |
| PD-1 | CT-011 with or without lenalidomide, ex vivo | MM | Enhanced NK cytotoxicity, additive effect of lenalidomide | 58 |
| PD-1 | CT-011 + tumor/DC vaccination, ex vivo | MM | Reduction in TREG number, enhanced T-cell response | 59 |
| PD-1 | DC vaccination with PD-L silencing, ex vivo | AML, CML | Enhanced alloreactive T-cell response | 105 |
| BTLA | Anti-BTLA, ex vivo | ALL, AML, CML, NHL, MM | Enhanced alloreactive T-cell response | 67 |
| CD200 | Anti-CD200, ex vivo | AML | Enhanced NK cytotoxicity | 84 |
| CD200 | Anti-CD200, ex vivo | CLL | Enhanced antigen-specific T-cell responses, reduction in Treg number | 85 |
| Molecule . | Therapy . | Tumor . | Outcome . | Reference . |
|---|---|---|---|---|
| CTLA-4 | Ipilimumab, in vivo | NHL | Two of 4 tumor regression, no increase in vaccine-specific T-cell responses, reduction in TREG number early after treatment; toxicity: mainly grade 1 or 2, 1 times grade 3 | 24 |
| CTLA-4 | Ipilimumab, in vivo | Relapsed/refractory B-cell NHL | Two of 18 clinical response, 5 of 16 enhanced T-cell response to recall Ag; toxicity: mainly grade 1 or 2, 6 of 18 grade 3 | 25 |
| CTLA-4 | Ipilimumab after alloSCT, in vivo | AML, CML, CLL, HL, NHL, MM | Three of 29 clinical response; toxicity: no induction of GVHD, 4 of 29 organ-specific immune adverse events | 29 |
| CTLA-4 | Anti–CTLA-4, ex vivo | HL | Abrogated TREG suppression | 17 |
| CTLA-4 | Anti–CTLA-4, ex vivo | CLL | Enhanced tumor-specific T-cell response | 109 |
| PD-1 | BMS-936,558, ex vivo | ALL, AML, CML, NHL, MM | Enhanced alloreactive T-cell response | 20 |
| PD-1 | Anti–PD-L1, ex vivo | NHL | Enhanced T-cell response | 52 |
| PD-1 | Anti–PD-L1 and anti–PD-L2, ex vivo | HL | Restored T-cell response | 53 |
| PD-1 | Anti–PD-L1, ex vivo | HCV lymphoma | Abrogated TREG suppression, reduction in Treg number | 55 |
| PD-1 | CT-011, in vivo | AML, CLL, HL, NHL, MDS, MM | Six of 17 clinical response, 1 complete remission, no toxicity | 57 |
| PD-1 | CT-011 with or without lenalidomide, ex vivo | MM | Enhanced NK cytotoxicity, additive effect of lenalidomide | 58 |
| PD-1 | CT-011 + tumor/DC vaccination, ex vivo | MM | Reduction in TREG number, enhanced T-cell response | 59 |
| PD-1 | DC vaccination with PD-L silencing, ex vivo | AML, CML | Enhanced alloreactive T-cell response | 105 |
| BTLA | Anti-BTLA, ex vivo | ALL, AML, CML, NHL, MM | Enhanced alloreactive T-cell response | 67 |
| CD200 | Anti-CD200, ex vivo | AML | Enhanced NK cytotoxicity | 84 |
| CD200 | Anti-CD200, ex vivo | CLL | Enhanced antigen-specific T-cell responses, reduction in Treg number | 85 |
ALL indicates acute lymphoid leukemia; CLL, chronic lymphoid leukemia; NHL, non-Hodgkin lymphoma; MDS, myelodysplastic syndrome; HCV, hepatitis C virus; and GI, gastrointestinal tract.
Because of their potent suppressive function, coinhibitory molecules became major targets of preclinical and clinical blocking studies. For example, in a murine thymoma model, CTLA-4 blockade after DC vaccination improved survival and resulted in a sustained increase in the number of antigen-specific T cells.23 In a phase 1 study that included 4 non-Hodgkin lymphoma patients, 2 subjects developed a clinical response on ipilimumab treatment.24 No enhanced T cell–mediated antitumor reactivity could be observed, although TREG levels decreased, suggesting that CTLA-4's effectiveness may be attributed to TREG depletion via antibody-dependent cell-mediated cytotoxicity. In a follow-up study with 18 non-Hodgkin lymphoma patients, ipilimumab administration resulted in clinical responses in 2 patients, and in several patients enhanced T-cell responses against KLH and tetanus toxoid were observed.25 Overall toxic effects were limited in these studies; and although durable responses were rare, the response rate resembled that of the first clinical trials in solid cancers. Because only small numbers of patients with hematologic malignancies have been treated so far, more research is warranted to draw conclusions.
Allogeneic T-cell function after alloSCT is also strongly influenced by coinhibitory molecules. The importance of CTLA-4 in modulating allogeneic immune responses has been confirmed by the association of certain CTLA-4 genotypes with the incidence of leukemia relapse and overall survival after alloSCT.26 Although not all functional consequences of reported polymorphisms have been elucidated, the CT60 single nucleotide polymorphism is postulated to influence the transcription of the sCTLA-4 variant, hampering normal CTLA-4 function.27 Interestingly, it was demonstrated that CTLA-4 blockade shortly after alloSCT increased GVHD in a CD28-dependent manner.28 However, when anti–CTLA-4 was administered at later time points after alloSCT, the GVT effect was boosted without signs of GVHD. Shortly after alloSCT, conditioning-related mucosal barrier injury, leading to a proinflammatory cytokine storm, tissue damage, and inflammation, may induce major T-cell activation in GVHD tissues. However, at later time points, these inflammatory events have diminished, and there is no general T-cell activation. In patients, ipilimumab administration at late time points after alloSCT has been explored in one phase 1 trial.29 After a single infusion of ipilimumab in 29 alloSCT patients with a recurrent or progressive hematologic malignancy, 3 clinical responses were observed. Importantly, no induction or exacerbation of clinical GVHD was reported, although, similar to other CTLA-4 blockade trials, 14% of the patients showed organ-specific immune adverse events. The lack of GVHD induction is probably attributed to the median interval of 1 year between last donor cell infusion and ipilimumab administration. This provides a window for antitumor immunotherapy in the posttransplantation setting and emphasizes the importance of appropriate timing.
PD-1
Expression and function of PD-1.
Programmed death-1 (PD-1; CD279) is another immunoreceptor belonging to the B7/CD28 family.30 In 1992, PD-1 was identified on hybridoma T cells undergoing apoptosis and was thought to be a programmed cell death-induced gene.31 Further characterization demonstrated that PD-1 is inducibly expressed on stimulated CD4+ T cells, CD8+ T cells, B cells, and monocytes.32 PD-1 binds 2 B7 family ligands, PD-L1 (B7-H1; CD274) and PD-L2 (B7-DC; CD273).33 Their interaction with PD-1 differs in affinity34 and type because of a conformational transition in PD-L1, but not PD-L2, on binding.35 Although PD-L2 expression is mainly restricted to APCs, such as DCs and macrophages, PD-L1 is expressed on many nonlymphoid tissues as well.36 Furthermore, multiple tumor types express PD-L1, and its expression is elevated after IFN-γ exposure.37 PD-L1 molecules on tumor cells can deliver negative signals toward PD-1–expressing tumor-reactive T cells, thereby inhibiting antitumor immunity.38 Indeed, PD-L1 expression has been associated with poor prognosis in solid tumors.37,39 Interestingly, PD-L1 is also able to bind CD80, mediating T-cell inhibition.40 In addition to downstream signaling of PD-L1,41 also engagement of PD-L2 resulted in T-cell inhibition, further illustrating the complexity of these interactions.42
It has been well demonstrated that PD-1 plays a crucial role in T-cell regulation in various immune responses, such as peripheral tolerance, autoimmunity, infection, and antitumor immunity.36 Elevated PD-1 expression on viral antigen-specific CD8+ T cells in chronic viral infections was recognized as a hallmark for T-cell dysfunction on antigen restimulation.43 This phenomenon known as exhaustion is characterized by the sequential loss of the ability to proliferate, secrete cytokines, and lyse target cells. Especially in HIV infection, T-cell impairment could be relieved by PD-1 blockade both in vitro and in animal models.44,45 Exhausted T cells have increased expression of multiple coinhibitory receptors and a distinct gene signature, different from anergic cells, resulting in changes in TCR and cytokine signaling pathways.46 Indeed, an exhaustion-specific gene signature, recently defined by Quigley et al,47 demonstrated that PD-1 downstream signaling effects play an important role in the exhaustion of HIV-specific T cells. Furthermore, they showed that the transcription factor BATF (basic leucine zipper transcription factor, ATF-like) appears essential for downstream PD-1 signaling. In addition to these signaling effects, the PD-1 gene itself is subject to epigenetic regulation, as increased PD-1 expression on activated CD8+ T cells results from demethylation of the Pdcd1 locus.48 During conversion to functional memory T cells, remethylation of Pdcd1 occurs, whereas in exhausted T cells the Pdcd1 regulatory region remains demethylated.
PD-1 in hematologic malignancies.
In addition to CTLA-4, PD-1/PD-L interactions were shown to be of importance in hematologic malignancies. For instance, PD-L1 overexpression enhanced MM invasiveness and rendered tumor cells less susceptible to cytotoxic T lymphocytes (CTLs).49 This effect was alleviated in PD-1 knockout mice or by anti–PD-L1 antibody treatment, demonstrating the importance of the PD-1/PD-L pathway in this process. This role of PD-1 was also confirmed in an AML model, and interestingly, PD-L1 expression was elevated on tumor cells in vivo compared with in vitro.50 In another report, increased levels of PD-L1 on MM cells together with enhanced PD-1 expression on exhausted T cells was demonstrated.51 As expected, in mice, PD-L1 blockade improved survival after autologous SCT and whole cell vaccination from 0% to 40%. In humans, PD-L1 expression was observed on non-Hodgkin lymphoma tumor cells, and blockade greatly enhanced cytokine production of autologous tumor-reactive T cells.52 Furthermore, it was shown that tumor cells of Hodgkin lymphoma (HL) patients can express both PD-L1 and PD-L2, and PD-1 expression was elevated on HL-infiltrating T cells.53 In addition, in this case, blockade of PD-Ls mediated increased cytokine secretion by the infiltrated T cells. Furthermore, long-term persistent murine leukemia cells were shown to sequentially up-regulate PD-L1 and CD80, thereby conferring protection against immune destruction.54 On PD-L1 or CTLA-4 blockade, CTL-mediated lysis of these persistent AML cells was improved. Similar to the link of CTLA-4 and TREG, an elevated number of TREG exhibiting high PD-1 expression was described in HCV-associated lymphoma.55 In addition to PD-1 expression on CD8+ T cells and TREG, PD-L1 expression on APCs was important for tumor persistence of murine AML. Combining PD-L1 blockade with TREG depletion showed superior efficacy in clearance of AML because of alleviation of PD-1–dependent TREG-mediated suppression.56
Although clinical PD-1 blockade has not been as extensively tested as ipilimumab for CTLA-4, multiple clinical grade antagonistic anti–PD-1 antibodies have been developed (ie, CT-011, BMS-936,558 and MK-3475; NCT01295827). Furthermore, 2 anti–PD-L1 antibodies, BMS-936,559 (NCT00729664) and MPDL2180A (NCT01375842), one anti–PD-L2 antibody (NCT00658892), and a PD-L2 fusion protein AMP-224 (NCT01352884) are being tested in phase 1 clinical trials. Three studies involving hematologic cancers were performed with CT-011. One phase 1 clinical trial was conducted in patients with various hematologic malignancies and showed a clinical response in 6 of 17 patients, with few adverse events.57 Although the CD4+ T-cell count was elevated in the treated patients, no additional evidence of T-cell activation was found. In a preclinical study, ex vivo treatment with CT-011 enhanced the functionality of NK cells against autologous primary MM cells.58 In addition, the drug lenalidomide down-regulated PD-L1, and an additive effect was shown by combining lenalidomide with CT-011, rendering this combination a promising therapy for MM patients. Another study examined whether PD-1 blockade improves the effectiveness of myeloma/DC vaccination therapy because it is known that both myeloma cells and myeloma/DC hybridomas highly express PD-L1.59 Indeed, ex vivo addition of CT-011 resulted in enhanced myeloma lysis by T cells as well as a reduction in the number of TREG. However, until now, the most promising effects have been obtained with the monoclonal human anti–PD-1 antibody BMS-936,558 (MDX-1106; ONO-4538). Administration to patients with solid tumors was well tolerated, and only one serious adverse event (inflammatory colitis) was reported.60 Follow-up reports presented at ASCO 2010 and GU ASCO 2011 showed that persistent clinical responses were observed in approximately 30% of patients with renal cell carcinoma, prostate cancer, melanoma, and lung cancer on treatment with repetitive doses of anti–PD-1 antibodies.61,62 The lack of strong toxic effects in this study holds promise that PD-1 blockade might have a more subtle effect than CTLA-4 blockade, thereby highlighting anti–PD-1 antibodies as interesting candidates for cancer therapy.
The role of PD-1 in alloSCT has been investigated both in mice and men. In 2 similar murine studies dissecting the role of alloantigens in GVT and GVHD reactivity, it was found that alloreactive T cells recognizing antigens on GVHD-prone tissues are driven into dysfunction and apoptosis.63 Furthermore, the interaction of nonhematopoietic cells with alloreactive T cells prevented the formation of proper alloreactive memory cells by exploiting the PD-1/PD-L pathway.64 This means that, in addition to the detrimental effect of GVHD as such, the beneficial GVT effect is hampered as alloreactive T cells become functionally impaired. Notably, PD-L1 blockade late after alloSCT may partly restore the GVT reactivity without inducing GVHD. Moreover, these results support the importance of targeting hematopoietic-restricted MiHA because these are solely expressed by hematopoietic tumor cells and residual healthy immune cells of the recipient, but not by GVHD-prone tissues. In one of the few studies investigating chronic myeloid leukemia (CML), using a retrovirus-induced CML model, it was demonstrated that tumor-specific T cells can become exhausted.65 In this model, consisting of PD-1+ tumor-specific T cells and PD-L1+ CML cells, exhaustion was overcome using either PD-1–deficient cells or anti–PD-L1 administration. We and others have investigated the role of PD-1 in GVT immunity in alloSCT patients. High PD-1 expression was observed on alloreactive CD8+ TEM cells that specifically recognize hematopoietic-restricted MiHA in myeloid leukemia patients.20 In agreement, Mumprecht et al showed that the total T-cell population from CML patients had elevated levels of PD-1.65 In addition, CD117+ progenitor AML cells displayed low levels of CD80 and CD86, whereas PD-L1 was highly expressed, especially under inflammatory conditions.20 Because these observations were made in alloSCT patients who relapsed after initial powerful MiHA-specific T-cell responses, we postulated that PD-1 expression is involved in T-cell exhaustion. By stimulation with MiHA-loaded DC ex vivo, we aimed at activating these PD-1+ MiHA-specific TEM cells; however, results were suboptimal, suggesting an impaired state. Importantly, on treatment with anti–PD-1 or anti–PD-L1 blocking antibodies, we were able to reinvigorate MiHA-specific TEM proliferation. Notably, the effect of PD-1 blockade on MiHA-specific TEM cells from relapsed patients compared with patients in long-term remission was significantly stronger, indicating the function of PD-1 in T-cell exhaustion and subsequent tumor immune evasion.
BTLA
Expression and function of BTLA.
B and T lymphocyte attenuator (BTLA), that is, CD272, was identified in 2003 as an inhibitory receptor with structural similarities to CTLA-4 and PD-1.66 BTLA is mainly expressed by immune cells, including T and B cells, DCs, and myeloid cells.67,68 In contrast to other B7/CD28 family members, BTLA binds a member of the tumor necrosis factor receptor superfamily, namely, herpesvirus entry mediator (HVEM).69 Although HVEM is part of an intricate signaling network as it has at least 4 additional binding partners that distinctively mediate T-cell responses (ie, CD160, LIGHT; for lymphotoxin-like, exhibits inducible expression, and competes with HSV glycoprotein D for HVEM, a receptor expressed by T lymphocytes), lymphotoxin-α (LT-α) and herpes simplex virus glycoprotein D.70 BTLA or CD160 signaling on HVEM binding results in T-cell inhibition.69,71 However, HVEM present on T cells acts as a costimulatory receptor,72 thereby constituting a bidirectional pathway. Interestingly, naive T cells express both HVEM and BTLA, and these molecules form a T cell–intrinsic heterodimer complex.73 Because of formation of this complex, HVEM is unavailable for extrinsic ligands, and no costimulatory signal is transduced. Studies in BTLA-deficient mice revealed a predisposition to experimentally induced autoimmune encephalomyelitis.66 Furthermore, these mice develop late-onset spontaneous autoimmune hepatitis-like disease and multiorgan lymphocyte infiltration,74 implying the involvement of BTLA in maintaining self-tolerance. In humans, persistent expression of BTLA was observed on EBV- and CMV-specific CD8+ T cells, negatively affecting T-cell function.75,76 Furthermore, in melanoma patients, high BTLA expression correlated with impaired tumor-specific T-cell function.12,75 These tumor-specific T-cell responses could be restored in vitro by interference with the BTLA-HVEM pathway in combination with vaccination therapy. In addition, coexpression of PD-1, BTLA, and T-cell immunoglobulin and mucin domain 3 (TIM-3) rendered melanoma-specific CD8+ T cells highly dysfunctional, which could be restored by combined blockade of all 3 coinhibitory molecules.77
BTLA in hematologic malignancies.
A single administration of agonistic anti-BTLA antibody directly after alloSCT in mice completely prevented GVHD, whereas this treatment did not hamper GVT responses.78,79 Moreover, when using an antibody that specifically blocked the interaction of HVEM with BTLA, but not LIGHT, GVHD was attenuated.80 This suggests that in this model the costimulatory function of HVEM was dominant over its coinhibitory activity. Because both HVEM and BTLA can be expressed by T cells, bidirectional signaling can occur. Therefore, combining specific blocking antibodies with cell-specific knockout models for these molecules will be necessary to further unravel the intricate interactions between HVEM, BTLA, CD160, and LIGHT. In addition, we investigated the effect of a BTLA-blocking antibody on MiHA-specific T-cell function in alloSCT patients.67 As shown for PD-1, we observed that BTLA was also highly expressed on MiHA-specific TEM cells. Moreover, in 7 of 11 patients, BTLA blockade resulted in increased outgrowth of MiHA-specific TEM cells of transplanted patients. Interestingly, in 3 patients, BTLA blockade effects were more prominent than those of PD-1, indicating that BTLA has a nonredundant function to PD-1; therefore, it holds promise in post-SCT therapies.
New coinhibitory players
In addition to the previously discussed molecules, CD200 receptor (CD200R), TIM-3, LAG3, and PD-1H/VISTA were recently shown to contribute to T-cell inhibition and/or exhaustion in hematologic cancers.
CD200R is an inhibitory receptor previously thought to be most important on myeloid cells but is also expressed in the lymphoid lineage, such as NK, CD4+, and CD8+ T cells, especially on stimulation.81 Its ligand, CD200 (OX2), is a glycoprotein expressed on a broad number of cell types, including solid tumors and hematologic malignancies.82,83 In addition to the previously discussed coinhibitory molecules, CD200R inhibits both T- and NK-cell functionality.84,85 Furthermore, CD200/CD200R interactions are involved in tumor immune evasion, as CD200 expression on AML cells promoted tumor growth in mice.86 Interestingly, patients with CD200+ AML cells displayed a lower number of activated NK cells, and the effect on NK functionality was correlated to CD200 expression on the leukemia cells.84 In concordance with this, blockade of CD200 enhanced IFN-γ release and cytotoxicity by NK cells. Moreover, CD200 blockade restored T-cell proliferation and tumor control by immune cells for human CD200+ chronic lymphocytic leukemia both in vitro85 and in a humanized mouse model.87 However, in a follow-up report, treatment with anti-CD200 antibody caused loss of T cell–mediated tumor control because of clearance of the T cells.88 This was attributed to antibody-dependent cell-mediated cytotoxicity of CD200+ T cells caused by the IgG1 variant of anti-CD200, which was not observed for the IgG4 isotype. Therefore, blocking strategies should be carefully designed before proceeding to the clinic.
In addition, the cosignaling receptor TIM-3 is expressed on Th1 CD4+ and CD8+ T cells, and is involved in coinhibition. In mice, the interaction of TIM-3 with its ligand galectin-9 was demonstrated to be inhibitory in autoimmune diseases and malignancies.89 Furthermore, in HIV90 and melanoma patients,91 dysfunctional T cells have been shown to coexpress TIM-3. In this regard, interference with TIM-3 signaling is an interesting treatment option, and enhanced tumor vaccine efficacy has been observed by TIM-3 blockade.92 Interestingly, both TIM-3 and PD-1 were expressed on a subset of exhausted CD8+ T cells in a murine AML model and expression increased during tumor progression.93 Although either TIM-3 or PD-L1 blockade alone was not sufficient to improve survival, a combination of the 2 antibodies decreased tumor burden and enhanced survival. Furthermore, in human lymphoma, an interesting role for TIM-3 has been described on tumor endothelium.94 TIM-3 expressed on these endothelial cells mediated impaired CD4+ T-cell responses, and thereby promoted lymphoma onset, growth, and dissemination. In contrast, a stimulatory role for TIM-3 and galectin-9 has been reported in the interaction of CD8+ T cells and DCs.95 This discrepancy is reflected in research investigating its mechanism of action, where T-cell receptor stimulation is enhanced on TIM-3 signaling.96 This might be explained by the fact that T-cell exhaustion could be caused by prolonged TCR signaling, and TIM-3 accelerates this process. Another explanation is that, depending on which ligand binds to TIM-3, different modes of signaling are initiated. Therefore, TIM-3 may act as either a costimulatory or a coinhibitory factor, similar to BTLA.
Lymphocyte-activation gene 3 (LAG3; CD223) is a coinhibitory receptor highly similar to CD4 and binds HLA class II molecules.97,98 Importantly, LAG3 was implicated to inhibit T-cell function in HL patients.97,99 LAG3 seems to be nonredundant from PD-1, as both are expressed on distinct populations of CD8+ T cells.100 Recently, it was shown that in mice PD-1 and LAG3 act synergistically in the onset of autoimmune diseases and tumor escape.101,102 Furthermore in HL, both TREG and LAG3+ CD4+ T cells were shown to be involved in tumor immune evasion because the expression of FoxP3 and LAG3 coincided with the impairment of tumor-specific T-cell responses.99 Therefore, LAG3 is an interesting candidate to combine with therapies that use TREG depletion or PD-1 blockade.
Recently, another immunoregulatory molecule with similarities to PD-1, as well as to PD-L1, was simultaneously discovered by 2 groups: PD-1H (PD-1Homolog)103 or VISTA (V-domain Ig suppressor of T-cell activation).104 This molecule is broadly expressed on hematopoietic cells and is up-regulated on APCs and T cells on activation. Mice treated with a single dose of PD-1H/VISTA antibody did not develop GVHD after alloSCT; however, the mechanism of action was not elucidated.103 Another study identified PD-1H/VISTA as an inhibitory ligand on APCs and tumor cells.104 Here, PD-1H/VISTA Ig-fusion protein conveyed a lasting negative signal to T cells, and expression of the protein on APCs suppressed T-cell proliferation. Importantly, PD-1H/VISTA expression on tumor cells resulted in diminished antitumor immunity. The human ortholog was determined on the genomic level; and because of the important role of this immunoregulatory molecule in GVHD and tumor escape, PD-1H/VISTA is anticipated to be a potential therapeutic target.
Future prospects
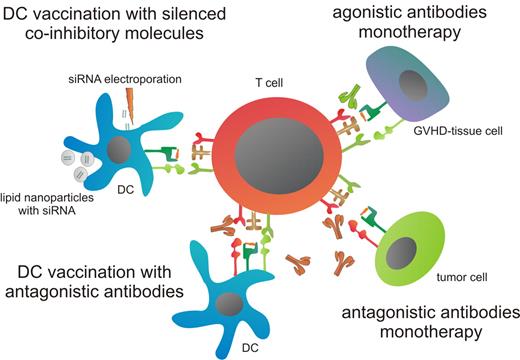
Several therapeutic strategies to interfere with the function of coinhibitory molecules are being explored to enhance antitumor T-cell immunity. The challenge of interference with immune checkpoints is to boost antitumor reactivity while avoiding systemic toxicity. This could potentially be achieved by (1) combining the alleviation of coinhibition with other therapeutic options, (2) blocking coinhibitory molecules that are intrinsically skewed toward antitumor responses rather than GVHD or autoimmune effects, and (3) optimal dosage and timing of antibody administration. Appealing combinations are the simultaneous targeting of multiple coinhibitory receptors or incorporation in existing cellular therapies. For example, DC vaccination may be applied together with blocking antibodies or siRNA knockdown of coinhibitory molecules to boost antitumor immunity or by administration of agonistic antibodies against coinhibitory molecules implicated in GVHD, adverse effects may be reduced (Figure 1).
Therapeutic strategies for interfering with coinhibitory molecules. First, blocking antibodies can be used to abrogate binding between coinhibitory molecules with the tumor-reactive T cell and tumor cells, thereby enhancing T-cell responses. In addition, DC therapy can be combined with antagonistic antibodies to boost the tumor-specific effect of DC vaccination. Another method to circumvent coinhibitory signaling during DC vaccination is silencing of coinhibitory molecules. Delivery of siRNA can be achieved either by electroporation or via lipid nanoparticles. Finally, coinhibitory molecules, which are differentially more involved in GVHD, can be stimulated by agonistic antibodies. This will attenuate GVHD-specific T-cell responses, thereby preventing attack of GVHD-prone tissues.
Therapeutic strategies for interfering with coinhibitory molecules. First, blocking antibodies can be used to abrogate binding between coinhibitory molecules with the tumor-reactive T cell and tumor cells, thereby enhancing T-cell responses. In addition, DC therapy can be combined with antagonistic antibodies to boost the tumor-specific effect of DC vaccination. Another method to circumvent coinhibitory signaling during DC vaccination is silencing of coinhibitory molecules. Delivery of siRNA can be achieved either by electroporation or via lipid nanoparticles. Finally, coinhibitory molecules, which are differentially more involved in GVHD, can be stimulated by agonistic antibodies. This will attenuate GVHD-specific T-cell responses, thereby preventing attack of GVHD-prone tissues.
Although anti–CTLA-4 and anti–PD-1 monotherapy have shown promising results, combination therapy with other treatment modalities, such as immunomodulatory anticancer agents, vaccines, or TREG depletion, is potentially necessary to effectively cure hematologic cancers. At the moment, several clinical trials are underway that target coinhibitory receptors in hematologic cancers. The effectiveness of CTLA-4 blockade by ipilimumab is investigated in lymphoma patients (NCT00047164), and the anti–PD-1 monoclonal antibody CT-011 is combined with 3 different therapies. In lymphoma patients, CT-011 is administered after autologous SCT (NCT00532259) and combined with rituximab (NCT00904722). Furthermore, the combination of CT-011 with a DC/AML fusion vaccine is being investigated as a therapy for AML patients (NCT01096602). Recently, we explored another treatment option in which an antigen-specific stimulation is combined with interference of coinhibition. We demonstrated that stimulation with PD-L1/L2 silenced MiHA-loaded DC boosted the expansion of MiHA-specific T cells ex vivo.105 After these promising results, we will start a clinical trial shortly combining DLI with vaccination of PD-L1/L2 silenced donor DCs loaded with hematopoietic-restricted MiHA (CCMO-trial no. NL37318). We think that these clinical studies provide a platform for incorporating blockade of coinhibitory molecules in adjuvant therapy of hematologic malignancies, with numerous options for combination therapies. Importantly, the risk of breaking tolerance systemically by blockade of one coinhibitory molecule could be prevented by using lower levels of multiple blocking antibodies targeting different inhibitory molecules simultaneously because together these may boost immune responses in a nonredundant manner. This is stressed by the fact that exhausted T cells are known to display multiple different coinhibitory receptors.106 By analyzing the downstream pathways of different coinhibitory receptors, one could limit these options and exclude combinations of receptors that have redundant effects, and focus on synergistic combinations. Notably, a clinical trial in solid tumors has started which combines blocking antibodies against PD-1 en CTLA-4 (NCT01024231), harnessing the power of these 2 nonredundant immune checkpoints.
The crux in alloSCT is the separation of GVT and GVHD reactivity. Although CTLA-4 and PD-1 clearly contribute to T-cell exhaustion, their activation might have too broad consequences early after alloSCT, and interfering with their signaling might deteriorate GVHD. Interestingly, anti-BTLA was recently reported to impair GVHD while allowing GVT reactions.78,107 Whether or not this important distinction in alloreactive responses also exists in humans needs to be evaluated, but it renders BTLA an important candidate for posttransplantation immunotherapy. Furthermore, the timing of coreceptor blockade seems to be essential to boost GVT without causing GVHD.28,64 Early after alloSCT, the patient is in a highly activated immunologic state because of chemotherapy-induced tissue damage and subsequent inflammation, especially in GVHD target tissues, such as skin and gut. To release immune checkpoints at this time would be dangerous because T cells would home to these inflamed alloantigen-expressing GVHD sites and destroy healthy cells. However, a delayed treatment window after alloSCT is possible, when there is no systemic “inflammatory” state. However, at the tumor site where the inflammation is sustained, antitumor MiHA-specific T cells may be specifically boosted, resulting in renewed GVT effects.
Altogether, coinhibitory molecules play a pivotal role in natural and immunotherapy-induced T cell–mediated immunity against hematologic cancers. With increasing knowledge of a growing number of coinhibitory molecules, novel mono- and combinatorial treatment options become available. In the end, this could lead to optimized immunotherapy against hematologic cancers, with limited risk of adverse events, such as the induction of autoimmune diseases and GVHD.
Acknowledgments
This work was supported by the Dutch Cancer Society (grants KWF 2008-4018 and KWF 2012-5041).
Authorship
Contribution: W.J.N., W.H., R.v.d.V., and H.D. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Harry Dolstra, Laboratory of Hematology, Department of Laboratory Medicine, Radboud University Nijmegen Medical Centre, Geert Grooteplein 8, PO Box 9101, 6500 HB Nijmegen, The Netherlands; e-mail: h.dolstra@labgk.umcn.nl.
References
Author notes
W.J.N. and W.H. contributed equally to this study.