Abstract
The MLL-partial tandem duplication (PTD) associates with high-risk cytogenetically normal acute myeloid leukemia (AML). Concurrent presence of FLT3-internal tandem duplication (ITD) is observed in 25% of patients with MLL-PTD AML. However, mice expressing either Mll-PTD or Flt3-ITD do not develop AML, suggesting that 2 mutations are necessary for the AML phenotype. Thus, we generated a mouse expressing both Mll-PTD and Flt3-ITD. MllPTD/WT:Flt3ITD/WT mice developed acute leukemia with 100% penetrance, at a median of 49 weeks. As in human MLL-PTD and/or the FLT3-ITD AML, mouse blasts exhibited normal cytogenetics, decreased Mll-WT-to-Mll-PTD ratio, loss of the Flt3-WT allele, and increased total Flt3. Highlighting the adverse impact of FLT3-ITD dosage on patient survival, mice with homozygous Flt3-ITD alleles, MllPTD/WT:Flt3ITD/ITD, demonstrated a nearly 30-week reduction in latency to overt AML. Here we demonstrate, for the first time, that Mll-PTD contributes to leukemogenesis as a gain-of-function mutation and describe a novel murine model closely recapitulating human AML.
Introduction
Acute myeloid leukemia (AML) is a genetically heterogeneous disease. Recurrent cytogenetic and molecular gene aberrations have been used to classify AML patients into distinct subsets that differ in biologic, clinical, and prognostic characteristics. The MLL gene, located at chromosome band 11q23, encodes for a protein involved in epigenetic regulation of gene expression.1 In AML, this gene is frequently involved in chromosome translocations at 11q23 and, at the molecular level, is fused with one of more than 50 different partners.2 We first reported an internal duplication of MLL, an in-frame repeat producing an elongated protein retaining all functional domains, in cytogenetically normal (CN)–AML.3 Approximately 5% to 7% of CN-AML patients have a MLL-partial tandem duplication (PTD) mutation, which is associated with unfavorable prognosis,3-5 but if and how it contributes to myeloid leukemogenesis remain to be elucidated. Approximately 25% of CN-AML patients with the MLL-PTD had constitutive activation of FLT3, a tyrosine kinase receptor regulating proliferation and survival of hematopoietic cells, via an internal tandem duplication (FLT3-ITD), and have a very poor prognosis.6 This suggests that both MLL-PTD and FLT3-ITD mutations are necessary for an AML phenotype, as supported by the broadly accepted 2-hit model.7 Indeed, a Mll-PTD mouse, created by knocking in exons 5 to 11 in-frame and driven off of the endogenous Mll promoter, did not develop AML.8 Similarly, the Flt3ITD/Wildtype(WT) or Flt3ITD/ITD knock-in mice do not develop AML.9 Thus, we crossed the heterozygous MllPTD/WT and Flt3ITD/WT knock-in mouse strains to create MllPTD/WT:Flt3ITD/WT double knock-in mice.8,9 This model, which develops AML, is the first that requires the MLL-PTD. In this model, the 2 mutated genes are regulated by their respective endogenous promoters to recapitulate the MllPTD/WT:Flt3ITD/WT AML found in humans. This differs from some other double-mutant mouse models of human AML that carry one mutation driven by the endogenous promoter and the other mutation driven by transgenes,10 or introduced via viral transduction,11 or 2 mutations driven off 2 endogenous promoters but requiring BM transplantation.12
Methods
Mouse strains
The MllPTD/WT, Flt3ITD/WT, and Flt3ITD/ITD mice were generated and genotyped as previously described.8,9 Male Flt3ITD/WT Balb/c mice were backcrossed onto the C57Bl/6J strain to purity and then bred with MllPTD/WT mice to generate MllPTD/WT:Flt3ITD/WT double knock-in offspring. Genotyping was performed as previously described.8,9 All animals studied were compared within litters and/or age- and sex-matched. All experiments were conducted under an approved The Ohio State University Institutional Animal Care and Use Committee protocol and following national guidelines and regulations.
Immunophenotypic analysis
For multiparameter flow cytometry, single-cell suspensions of BM and spleen were prepared from age- and sex-matched littermates. Red blood cells were lysed on ice with red blood cell lysis buffer (StemCell Technologies), washed in RPMI 1640 containing 10% FBS, resuspended in PBS with 0.5% FBS, and stained with the following monoclonal antibodies that were conjugated to V450, V500, FITC, PE, peridinin chlorophyll protein, allophycocyanin, or PE-Cy7: Gr-1, Mac-1, CD117, F4/80, CD3, B220, CD19, IgM, CD45.1, CD45.2, CD71, CD4, and CD8 (BD Biosciences) in PBS plus 0.5% FBS for 30 minutes. Analysis was performed using a BD LSRII cytometer or an Aria II cell sorter Version 6.1.2 (BD Biosciences) and FlowJo Version 7.6.5 (TreeStar) software programs. CD117+ cells were sorted into 50% FBS/RPMI 1640 for subsequent assays.
Pathologic examination, immunohistochemistry, and cytochemistry
Animals were monitored daily for the presence of disease by general inspection. White blood cell (WBC) counts and differentials were obtained biweekly from mice beginning at 35 weeks or when moribund. Peripheral blood was collected from maxillary vein puncture, and total and differential blood cell counts were determined using Hemavet 950 (Drew Scientific). Two consecutive WBC counts of more than 45 000 cells/μL together with an increase in neutrophil/lymphocyte ratio and/or morbidity defined as lethargy, enlarged spleen on palpation, and/or loss of 20% body weight were used as the criteria for death. Blood smears were prepared before death, followed by Wright-Giemsa staining. Killed animals were examined for the presence of tumors or other abnormalities, and organs were collected for further cell and histopathologic cellular analyses. Tissues were fixed for at least 72 hours in 10% neutral buffered formalin (Sigma-Aldrich), dehydrated in ethanol, cleared in xylene, and infiltrated with paraffin on an automated processor (Leica). For solid tissue samples, 4-μm-thick tissue sections were placed on charged slides, deparaffinized with xylene, rehydrated through graded alcohol washes, and stained with hematoxylin and eosin.
G-banded karyotype analysis
Cytogenetic analysis was done on BM cells obtained from the long bones of the animals. The cells were cultured overnight in RPMI 1640 medium with 2% L-Glutamine (Invitrogen), supplemented with 20% FBS (Hyclone Laboratories), and 2% penicillin and streptomycin (Invitrogen) at 37°C in an atmosphere of 5% CO2. Colcemid (Invitrogen) at a final concentration of 0.1 μg/mL was added for 1 hour. The cells were treated with hypotonic solution (0.075M KCl) for 15 minutes, fixed in 3:1 methanol:acetic acid, and dropped onto warm slides. Chromosomes were G-banded using trypsin and Wright stain by standard laboratory procedures. Twenty metaphases from each animal were completely analyzed.
Serial hematopathologic examinations, flow cytometric analysis, and serial transplantation
For transplantation, Ly5.2 spleen cells isolated from leukemic MllPTD/WT:Flt3ITD/WT mice were resuspended in PBS. Cells (5 × 106) were injected through the lateral tail vein into sublethally irradiated (450 cGy), syngenic Ly5.1 recipient mice. When moribund, mice were killed and hematopoietic tissues harvested and FACS analyses carried out. Spleen cells were serially transplanted up to 4 times.
Quantitative real-time RT-PCR
Predeveloped mouse TaqMan primer/probe sets for ActB (Mm01205647_g1), HoxA9 (Mm00439364_m1), and Flt3 (Mm00439016_m1) were purchased from Applied Biosystems. An allele-specific TaqMan assay for measuring mouse Mll-PTD and Mll-WT mRNA levels was performed as previously described.8 Real-time RT-PCR was carried out using the ABI 7900HT Fast Real-Time PCR System (Applied Biosystems). Relative and absolute quantification assays were carried out as previously described.13,14 Mouse Mll primer and probe sequences are available on request.
Statistical analyses
The log-rank test was used to evaluate overall survival between mouse groups, and Kaplan-Meier survival curves were used to display the results. Statistical significance of differences in parameters measured between WT, single knock-in mutant animals and double knock-in mutant mice were assessed using linear mixed-effects models if the data are correlated or ANOVA if the data are independent with P values less than .05 considered to be significant. Statistical analyses were conducted using SAS Version 9.2 (SAS Institute).
Results
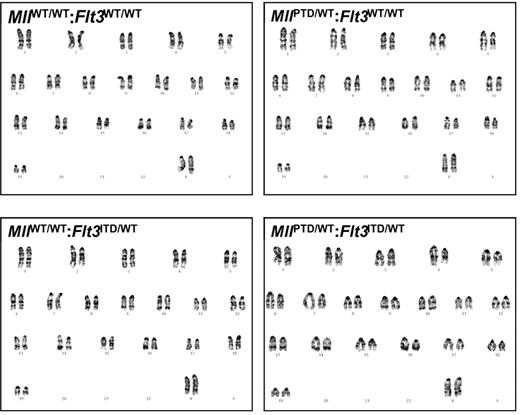
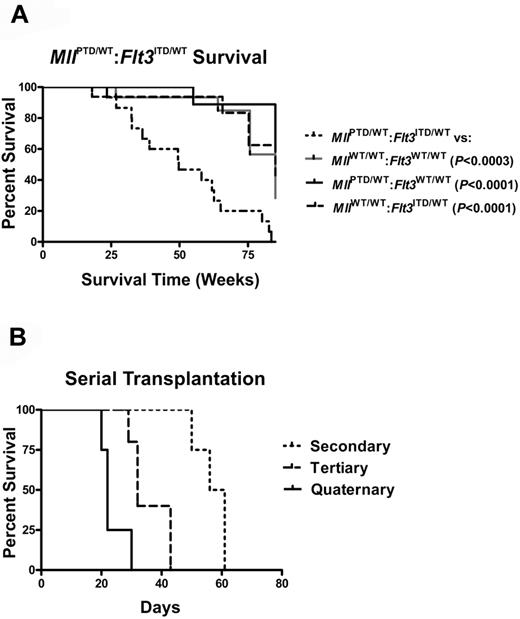
The double knock-in MllPTD/WT:Flt3ITD/WT mice developed acute leukemia with 100% penetrance. Seventy percent of the MllPTD/WT:Flt3ITD/WT mice developed 1 of 3 main subtypes of AML, as defined previously for murine leukemias (Figure 1A-B).15 The remaining mice developed biphenotypic (12%), B-cell (9%), or unclassifiable (9%) acute leukemia (Figure 1A). Compared with the single mutant knock-ins and WT mice, MllPTD/WT:Flt3ITD/WT mice developed significant leukocytosis (Figure 1C) and splenomegaly (Figure 1D-E). Additional criteria were met by the presence of more than or equal to 20% blasts in blood and blasts in nonhematopoietic organs, including liver and adrenal glands (Figure 1F), and thrombocytopenia and/or anemia (data not shown). Gross structural aberrations in chromosomes were not found with G-banding karyotype analysis of the age-matched single mutant knock-ins or in the leukemic double knock-in mice (Figure 2). MllPTD/WT:Flt3ITD/WT mice died with significantly reduced life spans and a median survival of 49 weeks versus 75 to 94 weeks for WT and single mutants (P < .0003; Figure 3A). Leukemia cells were transplantable; and with each serial passage of the cells, transplant recipients showed a progressively shorter period of time to overt morbidity resulting from AML (Figure 3B).
MllPTD/WT:Flt3ITD/WT mice develop a predominance of AML. (A) Frequencies of acute leukemia immunophenotypes identified in MllPTD/WT:Flt3ITD/WT mice (n = 33). (B) Representative flow cytometry plots from spleen and BM samples from age-matched controls and moribund MllPTD/WT:Flt3ITD/WT mice killed with elevated WBC counts. AML with maturation (myeloperoxidase [MPO+]/CD3−/CD19−/B220−/CD11b+/Gr1+/CD117−; 15%), AML without maturation group A (MPO+/CD3−/B220lo/CD19−/CD11blo/−/Gr1−/CD117+/−; 34%), AML without maturation group B (MPO+/CD3−/B220+/CD19−/CD11b+/−/Gr1−/CD117+/−; 21%). Regions highlighted in the red rectangle emphasize key differences in populations for each of the AML immunophenotypes. MPO staining by IHC and flow cytometry results for B-cell and biphenotypic leukemias are not shown. (C) WBC counts of leukemic MllPTD/WT:Flt3ITD/WT mice are significantly elevated at the time of death. Figure shows WBC counts from 3 age-matched cohorts of MllPTD/WT:Flt3ITD/WT mice with AML and controls. (D) MllPTD/WT:Flt3ITD/WT mice with leukemia exhibit significant splenomegaly at the time of death. Figure shows weights from 3 age-matched cohorts of MllPTD/WT:Flt3ITD/WT mice with AML and controls. (E) Representative image of spleens from a MllPTD/WT:Flt3ITD/WT mouse dying of AML with age-matched controls. (F) Representative blood smear stained with Wright-Giemsa and hematoxylin and eosin-stained organ preparations from a MllPTD/WT:Flt3ITD/WT mouse dying of AML. Micrographs demonstrate blasts in peripheral blood (original magnification ×1000) and extensive infiltrations of myeloid blasts in liver (original magnification ×400), and adrenal gland (original magnification ×400). Micrograph images were recorded using a Zeiss Axioskop, Olympus Magnafire digital camera, using a 10× eyepiece, 40×/0.65 NA Acroplan objective lens or 100×/1.40 NA Plan-Apochromat and Zeiss Axiovision Version 4.7.2 software.
MllPTD/WT:Flt3ITD/WT mice develop a predominance of AML. (A) Frequencies of acute leukemia immunophenotypes identified in MllPTD/WT:Flt3ITD/WT mice (n = 33). (B) Representative flow cytometry plots from spleen and BM samples from age-matched controls and moribund MllPTD/WT:Flt3ITD/WT mice killed with elevated WBC counts. AML with maturation (myeloperoxidase [MPO+]/CD3−/CD19−/B220−/CD11b+/Gr1+/CD117−; 15%), AML without maturation group A (MPO+/CD3−/B220lo/CD19−/CD11blo/−/Gr1−/CD117+/−; 34%), AML without maturation group B (MPO+/CD3−/B220+/CD19−/CD11b+/−/Gr1−/CD117+/−; 21%). Regions highlighted in the red rectangle emphasize key differences in populations for each of the AML immunophenotypes. MPO staining by IHC and flow cytometry results for B-cell and biphenotypic leukemias are not shown. (C) WBC counts of leukemic MllPTD/WT:Flt3ITD/WT mice are significantly elevated at the time of death. Figure shows WBC counts from 3 age-matched cohorts of MllPTD/WT:Flt3ITD/WT mice with AML and controls. (D) MllPTD/WT:Flt3ITD/WT mice with leukemia exhibit significant splenomegaly at the time of death. Figure shows weights from 3 age-matched cohorts of MllPTD/WT:Flt3ITD/WT mice with AML and controls. (E) Representative image of spleens from a MllPTD/WT:Flt3ITD/WT mouse dying of AML with age-matched controls. (F) Representative blood smear stained with Wright-Giemsa and hematoxylin and eosin-stained organ preparations from a MllPTD/WT:Flt3ITD/WT mouse dying of AML. Micrographs demonstrate blasts in peripheral blood (original magnification ×1000) and extensive infiltrations of myeloid blasts in liver (original magnification ×400), and adrenal gland (original magnification ×400). Micrograph images were recorded using a Zeiss Axioskop, Olympus Magnafire digital camera, using a 10× eyepiece, 40×/0.65 NA Acroplan objective lens or 100×/1.40 NA Plan-Apochromat and Zeiss Axiovision Version 4.7.2 software.
BM samples from MllPTD/WT:Flt3ITD/WT mice dying of AML are cytogenetically normal as are the age-matched, WT, and single-mutant controls. G-banded karyotype analysis of WT, single-mutant, and leukemic MllPTD/WT:Flt3ITD/WT mice was conducted on whole BM samples.
BM samples from MllPTD/WT:Flt3ITD/WT mice dying of AML are cytogenetically normal as are the age-matched, WT, and single-mutant controls. G-banded karyotype analysis of WT, single-mutant, and leukemic MllPTD/WT:Flt3ITD/WT mice was conducted on whole BM samples.
MllPTD/WT:Flt3ITD/WT mice develop a fatal AML that is transplantable. (A) Survival of WT and single knock-in (MllPTD/WT:Flt3WT/WT or MllWT/WT:Flt3ITD/WT) control mice and mice with the heterozygous alleles of MllPTD/WT:Flt3ITD/WT. (B) MllPTD/WT:Flt3ITD/WT cells from spleen containing AML are serially transplantable and lethal. Serially transplanted cells develop into progressively more aggressive AML with each successive passage.
MllPTD/WT:Flt3ITD/WT mice develop a fatal AML that is transplantable. (A) Survival of WT and single knock-in (MllPTD/WT:Flt3WT/WT or MllWT/WT:Flt3ITD/WT) control mice and mice with the heterozygous alleles of MllPTD/WT:Flt3ITD/WT. (B) MllPTD/WT:Flt3ITD/WT cells from spleen containing AML are serially transplantable and lethal. Serially transplanted cells develop into progressively more aggressive AML with each successive passage.
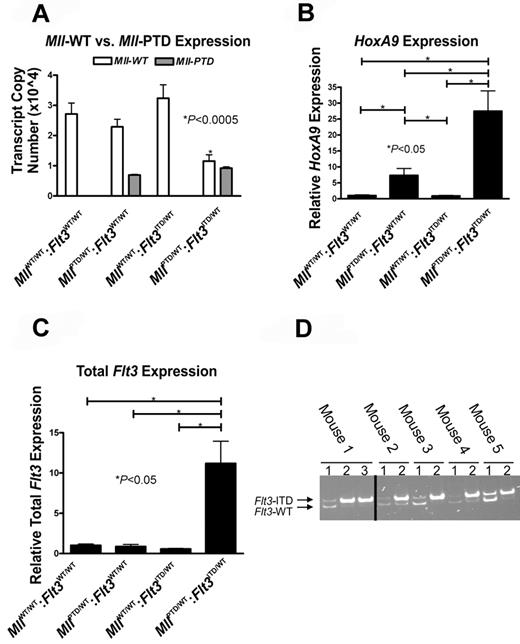
As reported previously for human MLL-PTD AML,14 we observed a nearly 60% decrease of the Mll-WT transcript copy number and a decrease in the Mll-WT-to-Mll-PTD ratio in the MllPTD/WT:Flt3ITD/WT AML mice compared with CD117+ BM samples from age-matched controls (Figure 4A). To obtain further support that the Mll-PTD is a gain-of-function allele,14,16 we assessed the impact on a known transcriptional target of Mll, the homeobox gene HoxA9. In leukemic MllPTD/WT:Flt3ITD/WT BM cells, HoxA9 expression was 25-fold higher (P < .05) versus similarly aged MllWT/WT:Flt3WT/WT mice and higher compared with the levels measured in the MllPTD/WT:Flt3WT/WT single knock-ins (P < .05; Figure 4B).
AML from MllPTD/WT:Flt3ITD/WT mice have molecular features similar to those reported for human MLL-PTD+ and/or FLT3-ITD+ AML. (A) Quantitative real-time RT-PCR was performed using CD117+ BM cells sorted to > 95% purity from age-matched mice ≥ 50 weeks of age to measure the copy numbers of the mouse Mll-PTD and the Mll-WT transcripts. Results demonstrate reduced Mll-WT copy number only in leukemic MllPTD/WT:Flt3ITD/WT mice. Figure is representative of 2 age-matched cohorts of mice. (B) Relative real-time RT-PCR measuring HoxA9 expression in whole BM cells from age-matched mice > 50 weeks of age. (C) Total Flt3 mRNA was measured by relative real-time RT-PCR using BM cells of age-matched mice ≥ 50 weeks of age. (D) Genomic PCR reactions using 150 ng DNA and genotyping primers to amplify both the Flt3-ITD and Flt3-WT loci were performed to demonstrate a reduction or loss of Flt3-WT in primary MllPTD/WT:Flt3ITD/WT AML samples taken from moribund mice. Multiple samples from each animal are defined as follows: lane 1, tail DNA from genotyping at 4 weeks; lane 2, whole BM DNA from the time of death when moribund with AML (50-80 weeks); and lane 3, sorted leukemic Ly5.2 MllPTD/WT:Flt3ITD/WT AML blasts from secondary transplant of MllPTD/WT:Flt3ITD/WT AML blasts into Ly5.1 recipients. The Ly5.2 → Ly5.1 adoptive transfer was used to obtain a pure population of MllPTD/WT:Flt3ITD/WT AML blasts for analysis. Five representative samples are shown. Reactions that did not produce any bands for Flt3-WT and Flt3-ITD were removed as noted by the black bar.
AML from MllPTD/WT:Flt3ITD/WT mice have molecular features similar to those reported for human MLL-PTD+ and/or FLT3-ITD+ AML. (A) Quantitative real-time RT-PCR was performed using CD117+ BM cells sorted to > 95% purity from age-matched mice ≥ 50 weeks of age to measure the copy numbers of the mouse Mll-PTD and the Mll-WT transcripts. Results demonstrate reduced Mll-WT copy number only in leukemic MllPTD/WT:Flt3ITD/WT mice. Figure is representative of 2 age-matched cohorts of mice. (B) Relative real-time RT-PCR measuring HoxA9 expression in whole BM cells from age-matched mice > 50 weeks of age. (C) Total Flt3 mRNA was measured by relative real-time RT-PCR using BM cells of age-matched mice ≥ 50 weeks of age. (D) Genomic PCR reactions using 150 ng DNA and genotyping primers to amplify both the Flt3-ITD and Flt3-WT loci were performed to demonstrate a reduction or loss of Flt3-WT in primary MllPTD/WT:Flt3ITD/WT AML samples taken from moribund mice. Multiple samples from each animal are defined as follows: lane 1, tail DNA from genotyping at 4 weeks; lane 2, whole BM DNA from the time of death when moribund with AML (50-80 weeks); and lane 3, sorted leukemic Ly5.2 MllPTD/WT:Flt3ITD/WT AML blasts from secondary transplant of MllPTD/WT:Flt3ITD/WT AML blasts into Ly5.1 recipients. The Ly5.2 → Ly5.1 adoptive transfer was used to obtain a pure population of MllPTD/WT:Flt3ITD/WT AML blasts for analysis. Five representative samples are shown. Reactions that did not produce any bands for Flt3-WT and Flt3-ITD were removed as noted by the black bar.
In agreement with previous findings that MLL-PTD patient blasts exhibit increased FLT3 expression,17 total Flt3 expression in BM of leukemic MllPTD/WT:Flt3ITD/WT mice also increased over time and was found to be 10-fold higher (P < .05) than controls (Figure 4C). Because the Real time RT-PCR conditions used did not distinguish between the Flt3-ITD and Flt3-WT transcripts, it was not clear if a selective increase in one or the other or both transcripts was occurring. In the human AML harboring FLT3-ITD, absence of the FLT3-WT allele at diagnosis occurs in approximately one-third of patients via a mechanism of uniparental disomy,18 and FLT3-ITD AML patients with an absent FLT3-WT allele at diagnosis have a worse prognosis as the result of more aggressive disease.13 We found that 9 of 10 MllPTD/WT:Flt3ITD/WT mice that were leukemia-free at 4 weeks of age carried the Flt3-WT allele. However, at the time of AML diagnosis, genomic PCR analyses of whole BM and transplantation-derived Ly5.2-sorted leukemic blasts revealed a dramatic reduction in Flt3-WT relative to Flt3-ITD or absence of the Flt3-WT allele (Figure 4D). The implication of these findings is that the “total Flt3” expression detected in the majority of leukemic samples is the result of up-regulation of expression of the remaining Flt3-ITD allele.
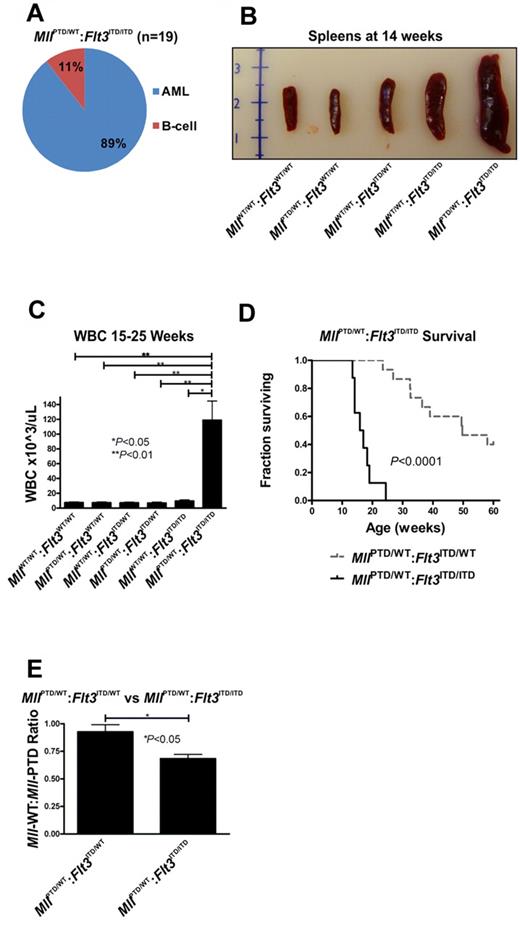
To test the contribution of this phenomenon (ie, loss of the Flt3-WT allele) to leukemogenesis, we crossed MllPTD/WT:Flt3ITD/WT mice with MllWT/WT:Flt3ITD/ITD mice to generate MllPTD/WT:Flt3ITD/ITD mice, which are homozygous for Flt3-ITD. MllPTD/WT:Flt3ITD/ITD mice had a higher incidence (89%) of AML compared with MllPTD/WT:Flt3ITD/WT mice (70%; Figure 5A vs Figure 1A). B-cell leukemia, which had been previously reported in one patient with MLL-PTD,19 developed in 11% of MllPTD/WT:Flt3ITD/ITD animals (Figure 5A). More than 95% of MllPTD/WT:Flt3ITD/ITD mice die with leukemic features similar to those described for MllPTD/WT:Flt3ITD/WT mice (Figure 5B-C). The disease was more aggressive with a significantly shorter median life span of MllPTD/WT:Flt3ITD/ITD mice compared with that of the MllPTD/WT:Flt3ITD/WT mice (19 vs 49 weeks, respectively; P < .0001; Figure 5D), supporting a dosage effect by the Flt3-ITD, as previously reported both in human and other mouse models.13,20,21
Acute leukemia in MllPTD/WT:Flt3ITD/ITD mice. (A) Only AML and B-cell leukemias developed in MllPTD/WT:Flt3ITD/ITD mice. Nineteen mice were analyzed. (B) MllPTD/WT:Flt3ITD/ITD mice with leukemia exhibited significant splenomegaly at time of death. (C) WBC counts (median ± SEM) for leukemic MllPTD/WT:Flt3ITD/ITD and age-matched control genotypes at the time of death. (D) MllPTD/WT:Flt3ITD/ITD mice died of AML with a significantly shorter latency than MllPTD/WT:Flt3ITD/WT mice. (E) Copy number ratio of Mll-WT:Mll-PTD was calculated for age-matched nonleukemic 15- to 25-week-old MllPTD/WT:Flt3ITD/WT and leukemic MllPTD/WT:Flt3ITD/ITD mice. Results are representative of 2 age-matched cohorts of mice.
Acute leukemia in MllPTD/WT:Flt3ITD/ITD mice. (A) Only AML and B-cell leukemias developed in MllPTD/WT:Flt3ITD/ITD mice. Nineteen mice were analyzed. (B) MllPTD/WT:Flt3ITD/ITD mice with leukemia exhibited significant splenomegaly at time of death. (C) WBC counts (median ± SEM) for leukemic MllPTD/WT:Flt3ITD/ITD and age-matched control genotypes at the time of death. (D) MllPTD/WT:Flt3ITD/ITD mice died of AML with a significantly shorter latency than MllPTD/WT:Flt3ITD/WT mice. (E) Copy number ratio of Mll-WT:Mll-PTD was calculated for age-matched nonleukemic 15- to 25-week-old MllPTD/WT:Flt3ITD/WT and leukemic MllPTD/WT:Flt3ITD/ITD mice. Results are representative of 2 age-matched cohorts of mice.
As in the older aged MllPTD/WT:Flt3ITD/WT leukemias, the Mll-WT-to-Mll-PTD transcript ratio was reduced in CD117+ BM cells from 15- to 25-week-old leukemic MllPTD/WT:Flt3ITD/ITD mice compared with that measured in age-matched, preleukemic MllPTD/WT:Flt3ITD/WT mice (P < .05; Figure 5E). In our model, dosage effects of Mll-PTD were not feasible to study because of embryonic lethality of the homozygous MllPTD/PTD mice.8 However, the expression data showing Mll-WT reduction in the leukemic samples regardless of the Flt3-ITD dosage are supportive that this phenomenon, reduced Mll-WT expression, contributes to the development of murine AML disease.
Discussion
Here we report the first animal model demonstrating that the Mll-PTD defect directly contributes to acute myeloid leukemogenesis in cooperation with a second mutation, the Flt3-ITD. First, compared with other in vivo modeling approaches, our model harbors both mutations in the germline, and each gene mutation is under the control of the respective normal endogenous promoter. Thus, expression of the mutant alleles would occur in the proper physiologic and temporal context during hematopoietic development and aging. Furthermore, the knock-in approach was pursued in our model to eliminate the potential for artifacts inherent with other methodologies, such as retroviruses, which induce nonphysiologic overexpression of the gene of interest and/or chromosomal integration of targeting constructs leading to activation of a proto-oncogene.22-24
To assess the relevance of this novel mouse model to human AML, we evaluated several phenotypic and molecular features for which published data exist for the human counterpart AML. The development of several AML subtypes in our double mutation knock-in model is consistent with earlier reports of the wide range of French-American-British morphologic subtypes of human AML that carry the MLL-PTD and/or FLT3-ITD.4,6,25 The diagnosis of AML in our model associates with significantly reduced life span, and the disease is also characterized by extensive extramedullary involvement and increased disease aggressiveness in serial transplantation assays. Similarly, the presence of MLL-PTD and/or FLT3-ITD in AML patients treated with cytarabine-based chemotherapy regimens associates with early relapses and reduced survival times compared with patients with AML lacking these defects.26-29
We previously reported that human leukemic blasts taken from patients with MLL-PTD CN-AML express the mutant MLL without concurrently expressing the MLL-WT from the nonaffected chromosome.14 This silencing is the result, at least in part, of epigenetic alterations within the 5′ regulatory region of the WT MLL allele.14 Likewise, at the molecular level in AML blasts from our MllPTD/WT:Flt3ITD/WT mice, the expression of the Mll-WT, but not the Mll-PTD, is consistently and significantly reduced. Notably, this is an acquired event, as the young, preleukemic MllPTD/WT:Flt3ITD/WT mice express Mll-WT transcript at levels comparable with those in the single heterozygous mutant MllPTD/WT and MllWT/WT control mice that do not develop AML. Whether this reduction in the WT Mll allele of leukemic mice is also related to altered epigenetics is under investigation, but considering that the leukemic BM samples of MllPTD/WT:Flt3ITD/WT mice remain karyotypically normal, one would consider this to be highly probable for at least a large fraction of the CD117+ BM cells studied.
Indeed, our model with its long latency period to overt disease lends itself to studying the repercussions and mechanisms of the Mll-PTD in the presence or absence of Mll-WT. In the future, this model will further our understanding of the Mll-PTD compared with Mll chimeric fusions, such as Mll-AF9, which appear to require Mll-WT for full, abnormal function involved in regulation of at least some target genes, such as HoxA9.30 Our results showing increased HoxA9 transcript levels in the leukemic MllPTD/WT:Flt3ITD/WT BM cells that exhibit reduced Mll-WT expression strongly supports a gain of function for the Mll-PTD that does not appear to require a full complement of Mll-WT to function at the HoxA9 gene.
Additional similarities to human FLT3-ITD–positive AML demonstrated by our model include loss of Flt3-WT expression. Based on our findings, Flt3-WT is not silenced by epigenetic mechanisms, as PCR using genomic DNA demonstrates loss of the Flt3-WT allele rather than a loss at the mRNA transcript level. Given the inbred nature of the mouse model presented, more detailed studies using our MllPTD/WT:Flt3ITD/WT crossed with another strain carrying divergent single nucleotide polymorphisms will be required to determine whether the loss of the Flt3-WT allele at the genomic level is the result of an intrachromosomal microdeletion or partial or full uniparental disomy. The information gleaned from studies, such as these, will probably provide important information affecting a greater number of patients, particularly the 30% of cytogenetically normal AML patients with the FLT3-ITD mutation.
In conclusion, the MllPTD/WT:Flt3ITD/WT double knock-in novel mouse model provides what we believe to be the first experimental proof that the MLL-PTD directly contributes to myeloid leukemogenesis, when concurrently present with a second mutation, such as FLT3-ITD. The MllPTD/WT:Flt3ITD/WT AML recapitulates its human AML counterpart with regard to several phenotypic, cytogenetic, and molecular features. Indeed, reduced Mll-WT expression, increased total Flt3 expression and loss of Flt3-WT in our mouse model are features that have been associated with human MLL-PTD and/or FLT3-ITD AML.13,27,28,31,32 In addition, the absence of gross structural chromosomal aberrations in the double knock-in mouse mimics the normal karyotypes at diagnosis that constitutes nearly 45% of human AML; both MLL-PTD and FLT3-ITD are most frequently present in human CN-AML.25,29,33,34 This study supports our previous findings that the MLL-PTD is a gain-of-function mutation.14,16 Despite the germline nature of the 2 mutations in our mouse model, the AML that develops does so with relatively long latency, implying that the reduction in Mll-WT expression and the loss of the Flt3-WT are important events, among others that remain to be defined, for full transformation. This model thus affords us the opportunity to carry out in-depth studies to elucidate the underlying mechanisms involved in leukemic transformation that must occur in a more physiologically relevant time-frame and cellular context. It also provides a feasible and relevant model to investigate not only novel leukemia prevention strategies, such as enhancement of innate and/or antigen-specific immunity via vaccination, but also opportunities to explore novel, targeted antileukemia therapeutics. For example, we can envision that the reduction in the Mll-WT expression levels along with the up-regulation of the Flt3-ITD concurrent with the loss/reduction of Flt3-WT should merit assessment of epigenetic modifying agents combined with tyrosine kinase inhibitors. This is a potential therapeutic strategy to combat MLL-PTD/FLT3-ITD–positive AML in our preclinical model as well as in patients with AML marked by these identical mutations. By virtue of its resemblance to the phenotypic, cytogenetic, and molecular features of human disease, this new AML model carrying the Mll-PTD mutation represents a useful tool for studying underlying mechanisms of leukemogenesis and for development and testing of novel target therapies to improve the outcome of this poor-risk AML subtype.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge The Ohio State University (OSU) Leukemia SPORE Cytogenetics Core and the OSUCCC Shared Resources: (Flow Cytometry, Comparative Pathology and Mouse Phenotyping, and Biostatistics).
This work was supported by National Cancer Institute grants CA089341 and CA009338 (to M.A.C.), CA102031 (to G.M.), CA140158 (to M.A.C. and G.M.), and CA113434 (to B.H.L.); T32 GM12453 (OUS College of Medicine Fellowship and Pelotonia Graduate Fellowship, to N.A.Z.), T32 CA009338 (to K.M.B.); and Pelotonia Undergraduate Fellowship (to R.F.S. and D.A.Y.).
National Institutes of Health
Authorship
Contribution: N.A.Z., K.M.B., S.P.W., R.F.S., E.H.A., G.G.M., D.A.Y., K.K.M., C.M., C.K., A.M.D., N.A.H., and B.H.L. performed the experiments; N.A.Z., K.M.B., S.P.W., R.F.S., A.M.D., B.H.L., G.H., G.M., and M.A.C. designed the experiments; N.A.Z., K.M.B., S.P.W., R.F.S., X.Z., D.J., A.M.D., N.A.H., B.H.L., G.H., G.M., and M.A.C. analyzed the data; S.P.W., A.M.D., G.M., and M.A.C. conceived the original project idea; and N.A.Z., S.P.W., G.M., and M.A.C. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael A. Caligiuri, Comprehensive Cancer Center, James Cancer Hospital and Solove Research Institute, 300 West 12th Ave, Suite 521B, Columbus, OH 43210; e-mail: michael.caligiuri@osumc.edu.
References
Author notes
N.A.Z., K.M.B., and S.P.W. contributed equally to this study.

![Figure 1. MllPTD/WT:Flt3ITD/WT mice develop a predominance of AML. (A) Frequencies of acute leukemia immunophenotypes identified in MllPTD/WT:Flt3ITD/WT mice (n = 33). (B) Representative flow cytometry plots from spleen and BM samples from age-matched controls and moribund MllPTD/WT:Flt3ITD/WT mice killed with elevated WBC counts. AML with maturation (myeloperoxidase [MPO+]/CD3−/CD19−/B220−/CD11b+/Gr1+/CD117−; 15%), AML without maturation group A (MPO+/CD3−/B220lo/CD19−/CD11blo/−/Gr1−/CD117+/−; 34%), AML without maturation group B (MPO+/CD3−/B220+/CD19−/CD11b+/−/Gr1−/CD117+/−; 21%). Regions highlighted in the red rectangle emphasize key differences in populations for each of the AML immunophenotypes. MPO staining by IHC and flow cytometry results for B-cell and biphenotypic leukemias are not shown. (C) WBC counts of leukemic MllPTD/WT:Flt3ITD/WT mice are significantly elevated at the time of death. Figure shows WBC counts from 3 age-matched cohorts of MllPTD/WT:Flt3ITD/WT mice with AML and controls. (D) MllPTD/WT:Flt3ITD/WT mice with leukemia exhibit significant splenomegaly at the time of death. Figure shows weights from 3 age-matched cohorts of MllPTD/WT:Flt3ITD/WT mice with AML and controls. (E) Representative image of spleens from a MllPTD/WT:Flt3ITD/WT mouse dying of AML with age-matched controls. (F) Representative blood smear stained with Wright-Giemsa and hematoxylin and eosin-stained organ preparations from a MllPTD/WT:Flt3ITD/WT mouse dying of AML. Micrographs demonstrate blasts in peripheral blood (original magnification ×1000) and extensive infiltrations of myeloid blasts in liver (original magnification ×400), and adrenal gland (original magnification ×400). Micrograph images were recorded using a Zeiss Axioskop, Olympus Magnafire digital camera, using a 10× eyepiece, 40×/0.65 NA Acroplan objective lens or 100×/1.40 NA Plan-Apochromat and Zeiss Axiovision Version 4.7.2 software.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/120/5/10.1182_blood-2012-03-415067/4/m_zh89991294230001.jpeg?Expires=1769538193&Signature=DJNN0VH0pqRfl7sLF4ckYWpDi3E3hjSILJUJFmCLzkpWDBFCNfVQLx5QDkuxoAJTw3Nzau5-J6oJokpOXnSraYJf1SurYcfKWmRS-Iufw5TS1hcKuKj9PofiZY~lr7YLSzGwgFfv9XRZJAp5zYPc2bgX2Fr0tH7ZOf94JpsRKKaJt1UFIp4flVyKIvrYo-x5I4dRRB-WASz4Iv2B0B31O0J9958sV0trAEUPefz7EtNFthQFgnxAU4jEWBwjOW8xXxmfeOs4t~xEOXuHDiDLkN-z7Awt1llM4HVeOgV40I994oZzR48Xcx2mX2K6NWtqtwd~XAh0udw78a7SycvTVA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)