Antiplatelet factor 4 (PF4) antibodies have an important role in the most frequent drug-induced immune disorder, heparin-induced thrombocytopenia (HIT). In this issue of Blood, Sachais and coworkers propose a new feature that may explain why only some anti-PF4 antibodies are pathogenic.1 In addition to epitope specificity–determining affinity and a high titer, the ability of antibodies to promote formation of their own target antigens seems to be a key factor for pathogenicity.
Sachais et al took advantage of 2 anti-PF4 monoclonal antibodies of the same antibody isotype (IgG2bκ). The one antibody, designated KKO, is well known as the only monoclonal antibody identified to date that preferentially recognizes PF4 in complex with heparin and activates platelets, hereby mimicking the reactivity pattern of anti-PF4/heparin antibodies isolated from sera of patients with HIT. KKO has been used extensively in a series of in vitro and in vivo experiments to unravel mechanisms of HIT. The other antibody, designated RTO, also recognizes PF4, but its binding is not influenced by the presence or absence of heparin.
Soon after characterization of PF4/heparin complexes as the main antigen in HIT, several individuals were identified who also had anti-PF4 antibodies in their plasma, which bound to PF4 independently of the presence of heparin (ie, their reaction profile parallels that of monoclonal antibody RTO); these antibodies are not associated with platelet activation.2 More recently it has been proposed that these heparin-independent anti-PF4 antibodies are relatively frequent in patients with antiphospholipid syndrome and systemic lupus erythematosus, sometimes leading to false positive HIT antigen assays.3
KKO binding to PF4/heparin complexes is inhibited to a far greater extent by platelet activating compared with nonactivating anti-PF4/heparin antibodies obtained from patients (∼ 80% vs 30%, information provided by Dr D. Cines, University of Pennsylvania), while RTO binding is not affected at all. This characteristic of KKO is already used in a commercial PF4/heparin antibody assay (HemosIL HIT-Ab(PF4-H); Instrumentation Laboratory), in which agglutination of PF4/polyvinylsulfonate-coated latex beads by KKO is inhibited in the presence of human anti-PF4/heparin antibodies.4 Thus, KKO and similar monoclonal antibodies will help to design assays with improved specificity for clinically relevant anti-PF4/heparin antibodies.
Besides this clinically relevant aspect, the present study by Sachais and colleagues has some interesting implications on the pathogenicity and the nature of the immune response in HIT. Assessment of antibody binding by enzyme immunoassays is very artificial and does not allow detailed interaction studies. These are, however, important to better understand the pathogenesis of HIT on a molecular level. In recent years, methods developed in nanophysics have been applied to better understand mechanisms of antigen generation and antibody-antigen interactions. For example, atomic force microscopy5 and transmission electron microscopy of rotary-shadowed PF4/heparin complexes6 revealed the structure of the antigenic PF4/heparin complexes. Sachais et al now use rupture force spectroscopy to assess antibody-antigen interactions at the single molecule level.1 This technology allows for detailed characterization of antibody-antigen interaction and may be instrumental in understanding why certain antibodies with specificity for the same molecule have different biologic effects, for example, under different shear stress within a specific environment.
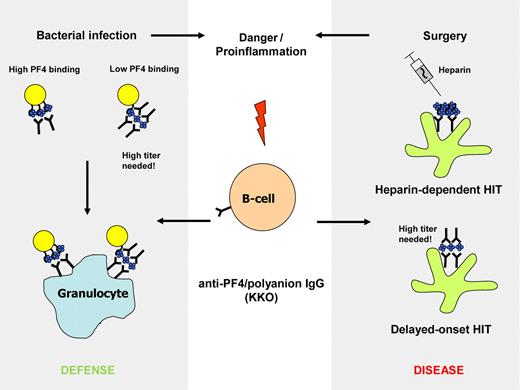
Sachais et al show that KKO also binds to PF4 alone, albeit with much lower affinity than to PF4/heparin complexes. More importantly, they found that KKO itself (but not RTO) is able to induce clustering of PF4, that is, it behaves somewhat like heparin, and induces oligomerization of PF4, thereby enhancing its own binding affinity. In other words, once its epitope is exposed on the molecule, KKO promotes generation of further epitopes by cross-linking PF4 tetramers. Heparin and other polyanions thereby augment the effect of the antibodies, creating a feed forward cycle. Although it has not been shown whether pathogenic anti-PF4/heparin antibodies isolated from patient sera also induce antigenic PF4 clusters, such a phenomenon would nicely reflect a feature of many acute-phase HIT antibodies, namely their ability to activate platelets even in the absence of heparin,7 a phenomenon that has been linked to delayed-onset HIT7 and delayed platelet-count recovery in HIT, in which the antibodies cause very strong platelet activation in the absence of any heparin (see figure).
The monoclonal anti-PF4/polyanion antibody KKO preferentially binds to PF4 clusters, as formed in PF4/heparin complexes during heparin treatment (right panel, top) and on bacteria with high PF4-binding capacity (left panel, top). This can lead to heparin-induced thrombocytopenia (HIT) and to opsonization of bacteria. If the antibodies are present in high titer, they can promote formation of their own epitope by clustering PF4. This might be an explanation for delayed-onset HIT (right panel, bottom) and could augment opsonization of bacteria with low PF4 binding capacity.
The monoclonal anti-PF4/polyanion antibody KKO preferentially binds to PF4 clusters, as formed in PF4/heparin complexes during heparin treatment (right panel, top) and on bacteria with high PF4-binding capacity (left panel, top). This can lead to heparin-induced thrombocytopenia (HIT) and to opsonization of bacteria. If the antibodies are present in high titer, they can promote formation of their own epitope by clustering PF4. This might be an explanation for delayed-onset HIT (right panel, bottom) and could augment opsonization of bacteria with low PF4 binding capacity.
The principle of inducing immunogenic clusters of PF4 by these antibodies may also have major implications for understanding other autoimmune disorders, as such antibodies could potentially induce a self-amplifying autoimmune disease by stabilizing antigenic determinants. While these findings by Sachais et al are potentially a major step toward understanding the pathogenesis of HIT, they are also important for interpretation of many of the previous studies performed with KKO. In these studies KKO by itself (in the absence of heparin) may also have induced some biologic effects.
The present study by Sachais and colleagues also nicely fits our recent proposal that HIT is a misdirected bacterial host defense.8 PF4 binds to bacteria and thereby exposes the epitope recognized by anti-PF4/heparin antibodies, which enhance phagocytosis of bacteria. In case of bacterial infection, some bacteria may have only weak PF4-binding capacity and escape opsonization/phagocytosis. In this case it would be a major advantage of these antibodies to be able to promote the danger signal by inducing formation of clustered PF4 (see figure).
It is also well known that anti-PF4/heparin antibodies have an unusually short persistence and, with very few exceptions, are no longer detectable within 100 days after acute HIT.9 In the view of the present study by Sachais et al and the capacity of KKO to self-promote formation of its epitope, it is biologically plausible that such antibodies are not produced by long-lasting memory B cells. Long-term persistence of high titers of these antibodies would bear the danger to cause continuous clustering of PF4.
We and others have also shown that normal individuals have anti-PF4/heparin antibodies in their plasma,10 likely induced by minor bacterial inflammation, such as periodontal disease. Under normal conditions, the pathologic-activating capacities of these antibodies are likely quenched by scavengers but when the capacity of these proposed scavengers is overloaded, for example, after major surgery and treatment with heparin, strong activation of platelets, monocytes, and endothelial cells result in a prothrombotic situation. This feature is reminiscent of many components of the innate immune system and is consistent with the view that the anti-PF4/heparin immune response is part of an old immune defense strategy that interfaces between the innate and the adaptive immune system. Further characterization of the unusual immune response toward PF4/polyanion complexes may help to better understand other pathogenic antibody-mediated immune reactions like autoimmunity. The present study by Sachais et al now adds the new concept that an antibody can promote its own binding epitope.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal