Individuals with β-thalassemia intermedia and hemoglobinopathies of equivalent severity who are infrequently or never transfused can still develop serious complications of iron overload. Taher et al report the first 1-year, randomized, double-blind, placebo-controlled trial of oral iron chelation therapy with deferasirox in this population.1
Until now deferasirox, which is approved for the treatment of patients with transfusional iron overload, has only been investigated in frequently or regularly transfused patients.2 Its use in those individuals with ineffective erythropoiesis and iron overload primarily because of increased intestinal absorption is unique.
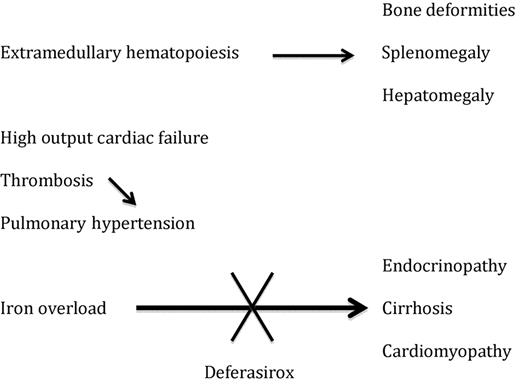
Appropriate care of patients with β-thalassemia major who are receiving regular blood transfusions includes monitoring of the iron burden and regular administration of iron chelation therapy starting early in life.3 Data from longitudinal studies indicates that with effective chelation therapy, complications of iron overload including endocrinopathies, liver disease, and cardiomyopathy can largely be avoided in most patients.4 Paradoxically, untransfused or infrequently transfused patients with β-thalassemia intermedia who have less severe hemoglobinopathies may suffer a spectrum of complications not experienced by their regularly transfused counterparts. These include high-output cardiac failure, pulmonary hypertension, hypercoagulability, and complications secondary to iron overload (see figure).5,6 The study by Taher et al provides high-quality data addressing the iron burden experienced by these individuals.
Complications in β-thalassemia intermedia and the potential role of deferasirox.
Complications in β-thalassemia intermedia and the potential role of deferasirox.
The trial randomized 166 patients at least 10 years of age or older to deferasirox 5 mg/kg, 10 mg/kg, or an equivalent placebo to each of these doses in a 2:1 manner. At baseline patients were required to have a liver iron concentration (LIC) of at least 5 mg Fe/g dw by a validated R2 magnetic resonance imaging methodology and a serum ferritin of at least 300 ng/mL, and they did not receive blood transfusions for at least 6 months, nor iron chelation for at least 1 month before enrollment. LIC was assessed at screening, 24 weeks, and 52 weeks. Dose increases were permitted after 24 weeks if an inadequate response had been achieved. Dose reductions were permitted for adverse effects.
These individuals with nontransfused or infrequently transfused thalassemia had very significant iron overload at baseline with median LIC and serum ferritin values of ∼ 12 mg Fe/g dw and 1000 ng/mL, respectively. For reference, LIC values of more than 7 mg Fe/g dw are associated with increased morbidity and values of more than 14 mg Fe/g dw are associated with increased mortality in β-thalassemia major.7 Removal of iron through phlebotomy was not generally possible in these thalassemia intermedia patients because of the anemia resulting from the underlying disorder of hemoglobin.
Both doses of deferasirox examined decreased LIC and ferritin compared with placebo with side effects that were relatively comparable with placebo. The only exception to this was a reversible mild increase in serum creatinine seen during the second 24 weeks of the study in 3 patients receiving deferasirox. Compliance with therapy was excellent at close to 95%, and 89% of patients completed the study. Nearly half of the patients had dose increases during the trial. Of note, in terms of efficacy, roughly 25% of patients randomized to 5 mg/kg and 50% of patients randomized to 10 mg/kg experienced at least a 30% decrease in LIC over the 52-week study period. The median reduction in serum ferritin of 202 ng/mL with deferairox 10 mg/kg, compared with an increase of 81 ng/mL with placebo, demonstrates effective removal of iron as measured by a more readily available clinical parameter.
Given the increased side effects experienced with higher doses of deferasirox in this trial and those that have been reported in other clinical trials, it is tempting to speculate that in this population, lower-intensity therapy for a more prolonged period may be the safest alternative. This issue will need to be addressed by future studies, including an ongoing extension of the current trial.
Besides demonstrating the safety and efficacy of oral iron chelation therapy with deferasirox in patients with nontransfusion dependent thalassemia, the study by Taher et al also has helped to better elucidate the side-effect profile of the drug. Because the inactive ingredients (excipients) were the same in both the active and placebo tablets, it is tempting to speculate that certain side effects such as nausea may be related to the excipients, rather than to the active ingredient. As the investigators note, further follow-up of this possibility is needed. Overall, this study provides important new data that may lead to a significant improvement in the care of patients with iron overload resulting from nontransfusion dependent hemoglobinopathies.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal