Abstract
Heparin-induced thrombocytopenia (HIT) is due primarily to IgG antibodies specific to platelet factor 4/heparin complexes (PF4/Hs) that activate platelets via FcγRIIA. CD148 is a protein tyrosine phosphatase that regulates Src kinases and collagen-induced platelet activation. Three polymorphisms affecting CD148 (Q276P, R326Q, and D872E) were studied in HIT patients and 2 control groups, with or without antibodies to PF4/Hs. Heterozygote status for CD148 276P or 326Q alleles was less frequent in HIT patients, suggesting a protective effect of these polymorphisms. Aggregation tests performed with collagen, HIT plasma, and monoclonal antibodies cross-linking FcγRIIA showed consistent hyporesponsiveness of platelets expressing the 276P/326Q alleles. In addition, platelets expressing the 276P/326Q alleles exhibited a greater sensitivity to the Src family kinases inhibitor dasatinib in response to collagen or ALB6 cross-linking FcγRIIA receptors. Moreover, the activatory phosphorylation of Src family kinases was considerably delayed as well as the phosphorylation of Linker for activation of T cells and phospholipase Cγ2, 2 major signaling proteins downstream from FcγRIIA. In conclusion, this study shows that CD148 polymorphisms affect platelet activation and probably exert a protec-tive effect on the risk of HIT in patients with antibodies to PF4/Hs.
Introduction
Heparin-induced thrombocytopenia (HIT) results from an atypical immune response to platelet factor 4/heparin complexes (PF4/Hs), with rapid synthesis of platelet-activating IgG antibodies that activate platelets via FcγRIIA receptors.1 The risk of HIT is probably dependent on the intensity of this immune response, because the likelihood of developing thrombocytopenia and thrombotic complications has been related to the plasma levels of IgG antibodies to PF4.2,3 However, the reasons explaining why only a subset of patients treated with heparin and who develop significant levels of IgG to PF4/Hs will present with HIT remain to be fully defined.
Protein tyrosine kinases (PTKs) and protein tyrosine phosphatases (PTPs) are crucial for the regulation of signaling pathways involved in the control of several cellular processes, including immune responses and platelet activation. Dysregulation of the equilibrium between PTK and PTP function can therefore have pathologic consequences.4,5 Among PTKs, Src family kinases (SFKs) have an important role in regulating immune responses and platelet adhesion, activation, and aggregation.6 SFKs are essential in the platelet FcγRIIA-dependent signaling pathway, and it was recently demonstrated that dasatinib, a SFK inhibitor, prevents platelet activation induced by HIT antibodies.7 In addition, SFKs are involved in collagen-dependent platelet activation via glycoprotein VI (GPVI).8 After collagen/GPVI interaction or FcγRIIA cross-linking, SFKs induce phosphorylation of conserved tyrosine residues within the immunoreceptor tyrosine–based activation motif, providing a docking site for the tyrosine kinase Syk. The specific interaction of Syk with the immunoreceptor tyrosine–based activation motif leads to its stimulation, an early key step of platelet activation via GPVI or FcγRIIA. Two specific inhibitors of Syk have recently been shown to inhibit FcγRIIA-dependent platelet activation induced by HIT antibodies.9,10
An important step in the activation of SFKs is the dephosphorylation of the inhibitory tyrosine residue in their C-terminal tail. Several soluble PTPs, including low molecular weight-PTP, PTP-1B, Src homology domain–containing inositol phosphatase-1, and phosphatase and tensin homolog deleted on chromosome 10, are involved in the regulation of collagen or FcγRIIA-dependent platelet activation.11-14 CD148 (also known as DEP-1), a receptor-like PTP, was recently shown to be critical for initiating GPVI signaling in platelets and positively regulating thrombus formation in mice.15 In addition, CD148 also contributes to the regulation of Fc receptor–mediated SFK activation in macrophages and neutrophils.16,17 CD148 is an R3 receptor-like protein tyrosine phosphatase with 8 fibronectin type III–like domains in its extracellular region, a single catalytic domain, and a cytoplasmic region with putative tyrosine phosphorylation sites. CD148 is encoded by PTPRJ, a gene located on chromosome 11, composed of 25 exons that contains several single-nucleotide polymorphisms (SNPs).18 More specifically, 3 nonsynonymous SNPs, that is, Q276P (rs1566734), R326Q (rs1503185), and D872E (rs4752904), located in the coding region have been identified in the British population.19
Given their central role in regulating SFKs and multiple signaling networks essential for immune cell function and platelet activation, we investigated whether CD148 might be involved in the pathogenesis of HIT. We hypothesized that a relationship may exist between polymorphisms of CD148 (ie, Q276P, R326Q, and D872E) and the development of heparin-dependent antibodies to PF4 and HIT. Because the frequency of homozygous CD148 276Q and 326R genotypes was found to be significantly higher in HIT patients than in controls, we then investigated whether platelet aggregation and signaling responses triggered by collagen and HIT antibodies varied according to CD148 genotypes.
Methods
HIT patients and control groups
The patient group (HIT) comprised 97 individuals (52 females and 45 males) with definite HIT. All had developed delayed onset thrombocytopenia, a significant decrease in platelet count after a median duration of heparin treatment of 10 days (range, 4-25 days), or both. Both PF4-specific ELISA (HAT; GTI) and serotonin release assay (SRA) were positive in every patient. However, different levels of antibodies to PF4 were measured in the plasma of HIT patients (median absorbance at 405 nm or A405 = 2.2; range, 0.76-4.0).
The first control group (Abneg) consisted of 179 patients (50 females and 129 males) who had undergone heart surgery with cardiopulmonary bypass (CPB). All patients had received high doses of unfractionated heparin during surgery, but none had developed an immune response to heparin after CPB because no significant level of PF4-specific antibodies was measured between 7 and 10 days postoperatively (median A405 = 0.13; range, 0.04-0.38; cut-off value = 0.4).
The second control group (Abpos) consisted of 160 patients (49 females and 111 males) who had also undergone cardiac surgery with CPB and had been treated by heparin postoperatively. All had developed significant levels of PF4-specific antibodies (median A405 = 1.13; range, 0.41-4.0) but without significant abnormal evolution in platelet count in the postoperative period.20 In addition, the SRA was consistently negative in every patient of this control group.
All patients and controls were whites and blood samples were collected after obtaining informed consent according to the Helsinki declaration principles. Moreover, both our institutional ethics committee and the Ministry of Research had previously approved collection of DNA from HIT patients and healthy controls for genetic studies (agreements DC2008-308 and 2011-N7).
Materials
Mouse monoclonal anti-CD9 (clone ALB6) and isotype control IgG1 were obtained from Acris Antibodies; monoclonal anti-GPIIb antibody (clone PL2-49) was kindly provided by Dr C. Kaplan (Institut National de la Transfusion Sanguine, Paris, France). Monoclonal anti-CD32 antibody (clone IV.3) was purchased from StemCell Technologies. Carboxyfluorescein-conjugated mouse monoclonal anti–human CD148 and isotype control were obtained from R&D Systems Europe. Monoclonal anti–phospho-tyrosine antibody (clone 4G10) was from Millipore. Antibodies against phospho-Lyn (tyrosine 507), phospho-Src (tyrosine 416), phospho-Linker for activation of T cells (tyrosine 191), and peroxidase-conjugated secondary antibodies were obtained from Cell Signaling Technology. Antibodies against phospho-PLCγ2, PLCγ2, Src, and Lyn were obtained from Santa Cruz Biotechnology.
Horm collagen and thrombin were obtained from Nycomed and Stago, respectively, and unfractioned heparin (Heparin Choay) was obtained from Sanofi. Dasatinib (Sprycel) was kindly provided by Bristol-Myers Squibb. Tirofiban (Agrastat) was obtained from Iroko Cardio United Kingdom. All other antibodies and reagents were purchased from Sigma-Aldrich unless otherwise indicated.
Genotyping
Genomic DNA was extracted from citrated whole blood using the FlexiGen DNA kit (QIAGEN) according to the manufacturer's instructions. Genotypes of CD148 Q276P (1176A/C; rs1566734), R326Q (1326G/A; rs1503185), and D872E (2965C/G; rs4752904) polymorphisms were defined by the PCR-high-resolution melting method using specific primers (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). PCR-high-resolution melting assays were performed in a 10-μL reaction volume containing the high-resolution melting master mix (Roche) 1× concentrate, We set up 10 ng of DNA and 3 pmol of each primer. PCR was set up in a LightCycler 480 (Roche), and the thermal cycling conditions were 10 minutes at 95°C, followed by 45 cycles of 10 seconds at 95°C, 10 seconds at 60°C, and 10 seconds at 72°C. The amplification cycle was followed by 1 minute at 95°C and 1 minute at 40°C, and melting was monitored from 60 to 95°C (0.02°C/second) with continuous fluorescence acquisition.
FcγRIIA H131R polymorphism (rs1801274) was studied as described previously.21
Expression level of CD148 in platelets
Washed platelets of healthy donors, carriers (n = 5) and noncarriers (n = 5) of the CD148 276P/326Q polymorphisms were incubated for 20 minutes either with labeled anti-CD148 antibody or isotypic control and then analyzed using an FACSCalibur flow cytometer and Kaluza analysis Version 1.1 software (BD Biosciences).
Platelet aggregation tests
Whole blood from 33 healthy aspirin-free donors was collected in acid-citrate-dextrose supplemented with prostaglandin E1 (0.1μM), and platelet-rich plasma was isolated by centrifugation at 150g for 15 minutes at 20°C). Platelets were washed and resuspended at a final count adjusted to 350 × 109/L.22 All reactions were performed at 37°C in an APACT 4 aggregometer (ELITech Group) with stirring at 1000 rpm.
Platelet aggregation tests with collagen and thrombin were performed with 225 μL of adjusted platelet suspension mixed either with 25 μL of collagen (final concentrations of 10, 1, and 0.5 μg/mL) or 25 μL of thrombin (0.05 IU/mL). In addition, FcγRIIA-dependent platelet aggregation was evaluated using murine monoclonal antibodies ALB6 (2.5 μg/mL) and PL2-49 (7.5 μg/mL) as described previously.23,24
Specific platelet aggregation tests were performed in the presence of dasatinib, an SFK inhibitor. In brief, washed platelets were preincubated for 10 minutes with dasatinib at final concentrations of 0, 20, 40, 60, 80, and 100nM before addition of either collagen (1 μg/mL) or ALB6 (2.5 μg/mL).
Finally, heparin-induced platelet aggregation (HIPA) also was studied with the plasma of 3 patients previously identified as having developed definite HIT. Importantly, both HIPA and SRA were positive with the plasma of each of these patients when tested with platelets from random donors. For each experiment, platelet suspensions (135 μL) from donors heterozygous for the FcγRIIA H131R polymorphism were incubated with 0.5 IU of heparin (25 μL) and HIT plasma (90 μL) and aggregation was continuously monitored for at least 20 minutes. Before testing, each HIT plasma was heated at 56°C for 1 hour and centrifuged to remove any residual thrombin activity.
Kinetics of phosphorylation
The PT phosphorylation pattern was analyzed after incubating platelet suspensions with collagen (0.5 μg/mL) for 45, 60, and 90 seconds or with ALB6 (2.5 μg/mL) for 90, 120, 150, and 600 seconds. Platelet activation was induced in the presence of Tirofiban (200μM) and stopped by addition of 5× lysis buffer (225mM Tris, pH 6.8, 50% glycerol, 5% sodium dodecyl sulfate, 0.25M DTT, 5mM EGTA, and 0.05% bromphenol blue). Platelet lysates were then heated at 100°C for 3 minutes and placed on ice for 10 minutes before being frozen at −20°C. Lysates were then submitted to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred onto nitrocellulose; tyrosine-phosphorylated proteins were detected by immunoblotting with specific antibodies. Quantitative phosphorylation analysis was performed by densitometric analysis and normalized with pan-lyn and pan-src signals. This procedure was applied with platelets collected from 6 separate donors, that is, 3 276QP/326RQ heterozygotes and 3 276QQ/326RR homozygotes.
Statistical analysis
A χ2 analysis was used to compare the frequencies of alleles and genotypes in HIT patients and controls. Considering the minor allele frequency for each SNP and the number of subjects studied in each group, only very few patients were identified as homozygous CD148 276PP or 326QQ. Therefore, we compared carriers of the 276P or 326Q allele (heterozygous + homozygous) to nonmutated subjects.
A Mann-Whitney U test or t test was performed to compare aggregation parameters and kinetics of phosphorylation in relation to CD148 polymorphisms. P values < .05 are considered significant.
Results
Genotype analysis of CD148
We analyzed 3 SNPs affecting the PTPRJ gene encoding of CD148, Q276P, R326Q, and D872E. The results presented in Table 1 show that the frequency of CD148 genotype 276QQ was significantly higher in HIT patients (83.5%) than in the Abneg and Abpos groups (65%, P = .001 and 70.5%, P = .03, respectively). Therefore, the 276P allele seemed to provide protection from the risk of HIT (odds ratio [OR] 0.47; 95% confidence interval [CI] 0.29%-0.94%, P = .03) in patients with antibodies to PF4/Hs, but it was not associated with the antibody response (frequency of the 276P allele = 19% and 16% in Abneg and Abpos groups, respectively). Analysis of the CD148 R326Q polymorphism demonstrated that the 326RR genotype was also more frequent in HIT patients (82.5%) than in Abneg (63.5%, P = .002) and Abpos controls (70%, P = .04). In addition, a strong linkage disequilibrium was evidenced between the Q276P and R326Q SNPs (D′ = 0.94, PHASE Version 2.1 software). It is also noteworthy to mention that no difference in the genotype frequencies was observed according to gender in the 3 groups studied. Alternatively, patients homozygous for the CD148 872E allele were more frequent in the HIT group compared with the Abpos and Abneg groups (19.5% vs 11% and 13.5%, respectively), but the difference was not significant, and the allele frequency was similar in the 3 groups of patients studied (Table 1).
Genotypes and MAF of CD148 polymorphisms in control subjects and HIT patients
| . | Abneg (n = 179) . | Abpos (n = 160) . | HIT (n = 97) . | OR (95% CI) . | P* . |
|---|---|---|---|---|---|
| Q276P (rs1566734) | |||||
| Genotypes n (%) | |||||
| 116 (65) | 113 (70.5) | 81 (83.5) | 0.36 (0.20-0.67)* | .001* | |
| QP + PP† | 63 (35) | 47 (29.5) | 16 (16.5) | 0.47 (0.25-0.89)** | .03** |
| MAF | |||||
| P | 0.19 | 0.16 | 0.09 | 0.41 (0.23-0.73)* | .003* |
| 0.52 (0.29-0.94)** | .03** | ||||
| R326Q (rs1503185) | |||||
| Genotypes n (%) | |||||
| RR | 114 (63.5) | 112 (70) | 80 (82.5) | 0.37 (0.20-0.68)* | .002* |
| RQ + QQ† | 65 (36.5) | 48 (30) | 17 (17.5) | 0.50 (0.27-0.92)** | .04** |
| MAF | |||||
| Q | 0.205 | 0.16 | 0.10 | NS | |
| D872E (rs4752904) | |||||
| Genotypes n (%) | |||||
| DD | 58 (32.5) | 59 (37) | 39 (40.2) | NS | |
| DE | 97 (54) | 83 (51) | 39 (40.2) | ||
| EE | 24 (13.5) | 18 (11) | 19 (19.5) | ||
| MAF | |||||
| E | 0.405 | 0.37 | 0.395 | NS |
| . | Abneg (n = 179) . | Abpos (n = 160) . | HIT (n = 97) . | OR (95% CI) . | P* . |
|---|---|---|---|---|---|
| Q276P (rs1566734) | |||||
| Genotypes n (%) | |||||
| 116 (65) | 113 (70.5) | 81 (83.5) | 0.36 (0.20-0.67)* | .001* | |
| QP + PP† | 63 (35) | 47 (29.5) | 16 (16.5) | 0.47 (0.25-0.89)** | .03** |
| MAF | |||||
| P | 0.19 | 0.16 | 0.09 | 0.41 (0.23-0.73)* | .003* |
| 0.52 (0.29-0.94)** | .03** | ||||
| R326Q (rs1503185) | |||||
| Genotypes n (%) | |||||
| RR | 114 (63.5) | 112 (70) | 80 (82.5) | 0.37 (0.20-0.68)* | .002* |
| RQ + QQ† | 65 (36.5) | 48 (30) | 17 (17.5) | 0.50 (0.27-0.92)** | .04** |
| MAF | |||||
| Q | 0.205 | 0.16 | 0.10 | NS | |
| D872E (rs4752904) | |||||
| Genotypes n (%) | |||||
| DD | 58 (32.5) | 59 (37) | 39 (40.2) | NS | |
| DE | 97 (54) | 83 (51) | 39 (40.2) | ||
| EE | 24 (13.5) | 18 (11) | 19 (19.5) | ||
| MAF | |||||
| E | 0.405 | 0.37 | 0.395 | NS |
MAF indicates minor allele frequency.
P values and ORs were calculated for genotype and allele frequencies between *Abneg and HIT and **Abpos and HIT.
Only few patients were homozygous 276PP or 326QQ. Therefore, we compared carriers of the 276P or 326Q allele (heterozygous + homozygous) to nonmutated subjects.
Expression level of CD148 in platelets
The surface expression level of CD148 measured by flow cytometry was identical in platelets heterozygous for the 276P/326Q alleles (mean relative fluorescence units = 5.99, SD = 0.3, n = 5) and cells isolated from noncarriers (mean relative fluorescence units = 6.04, SD = 0.7, n = 5).
Platelet aggregation according to CD148 polymorphisms
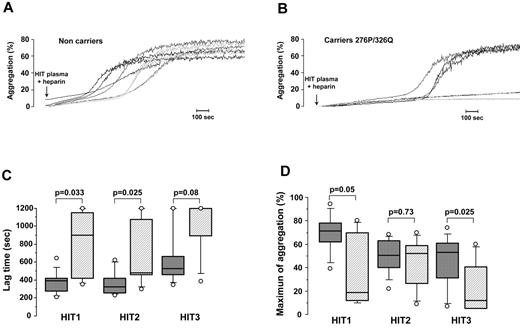
The apparent protective effect of CD148 276P and 326Q alleles on the risk of HIT suggested they could have an impact on platelet activation by anti-PF4/H antibodies. We therefore performed HIPA assays by testing the effects of plasma from 3 well-defined HIT patients on genotyped platelets in the presence of 0.5 IU/mL heparin. To avoid any possible influence of the FcγRIIA 131H/R polymorphism, only platelets isolated from heterozygous donors were studied. Representative results of HIPA are presented in Figure 1A and B. Rapid and significant aggregation was obtained with platelets from 276QQ/326RR subjects, whereas longer lag times were recorded when platelets from carriers of the 276P/326Q alleles were tested, and with some donors no aggregation was observed. Median lag time was therefore longer with platelets from donors expressing the 276P/326Q alleles whatever the HIT plasma tested (HIT1 = 684 vs 386 seconds with 276QQ/326RR platelets; P = .033, HIT2 = 479 vs 317 seconds; P = .025 and HIT3 = 1200 vs 650 seconds; P = .08; Figure 1C). In addition, the maximum aggregation induced by HIT1 and HIT3 plasma was also significantly lower with 276P/326Q platelets (41.5% vs 75% with 276QQ/326RR platelets; P = .05 and 13.5% vs 55%; P = .025 with HIT1 and HIT3 plasma, respectively; Figure 1D).
Influence of CD148 polymorphisms on platelet aggregation induced by HIT antibodies. (A-B) Representative platelet aggregation curves obtained with washed platelets from noncarriers (A) and carriers of the CD148 276P/326Q alleles (B) incubated with HIT plasma in the presence of unfractionated heparin (0.5 IU/mL). All donors tested were FcγRIIA 131H/R heterozygotes. (C-D) Lag time (C) and maximum aggregation (D) obtained with 3 different HIT plasma samples in relation to Q276P/R326Q polymorphisms (▩ noncarriers n = 10 and ▨ carriers of 276P/326Q alleles n = 7). All donors tested were FcγRIIA 131H/R heterozygotes. The horizontal lines in the middle of box-and-whiskers plots show the median values. The tops and bottoms of boxes show the 25th and 75th percentiles. The whiskers define the 10th and 90th percentiles. Mann-Whitney U test was performed to compare aggregation parameters in relation to polymorphisms of CD148, and P values are displayed.
Influence of CD148 polymorphisms on platelet aggregation induced by HIT antibodies. (A-B) Representative platelet aggregation curves obtained with washed platelets from noncarriers (A) and carriers of the CD148 276P/326Q alleles (B) incubated with HIT plasma in the presence of unfractionated heparin (0.5 IU/mL). All donors tested were FcγRIIA 131H/R heterozygotes. (C-D) Lag time (C) and maximum aggregation (D) obtained with 3 different HIT plasma samples in relation to Q276P/R326Q polymorphisms (▩ noncarriers n = 10 and ▨ carriers of 276P/326Q alleles n = 7). All donors tested were FcγRIIA 131H/R heterozygotes. The horizontal lines in the middle of box-and-whiskers plots show the median values. The tops and bottoms of boxes show the 25th and 75th percentiles. The whiskers define the 10th and 90th percentiles. Mann-Whitney U test was performed to compare aggregation parameters in relation to polymorphisms of CD148, and P values are displayed.
FcγRIIA-dependent platelet aggregation also was studied using the monoclonal antibodies ALB6 and PL2-49, with platelets isolated from healthy donors heterozygous for the FcγRIIA 131H/R dimorphism. The lag time measured with 276P/326Q platelets was significantly longer compared with that recorded with 276QQ/326RR platelets (median = 133 vs 101 seconds, P = .05 and 136 vs 84 seconds; P = .035 with ALB6 and PL2-49, respectively; Figure 2A). However, the maximum aggregation was similar whatever the CD148 genotype (median = 75% vs 76% for ALB6 and 73% vs 73% for PL2-49, respectively; Figure 2B). In addition, no significant difference in the lag time or maximum aggregation was found in relation to the CD148 D872E polymorphism (supplemental Figure 1).
Collagen- and FcγRIIA-dependent platelet aggregation according to CD148 Q276P/R326Q polymorphisms. (A-B) Washed platelets isolated from healthy heterozygous FcγRIIA 131H/R donors (n = 20) were incubated with murine IgG1 monoclonal antibodies ALB6 (2.5 μg/mL) and PL2-49 (7.5 μg/mL). Lag time (A) and maximum aggregation (B) were analyzed according to CD148 polymorphisms (▩ noncarriers = 13 and ▨ carriers of the 276P/326Q alleles = 7). (C-D) Washed platelets isolated from healthy donors (n = 33) were incubated with 0.5, 1, or 10 μg/mL collagen or with 0.05 IU/mL thrombin. Lag time (C) and maximum aggregation (D) were analyzed in relation to CD148 polymorphisms (▩ noncarriers = 21 and ▨ carriers of the 276P/326Q alleles = 12). The horizontal lines in the middle of box-and-whiskers plots show the median values. The tops and bottoms of boxes show the 25th and 75th percentiles. The whiskers define the 10th and 90th percentiles. Mann-Whitney U test was performed to compare aggregation parameters in relation to polymorphisms of CD148, and significant P values are displayed.
Collagen- and FcγRIIA-dependent platelet aggregation according to CD148 Q276P/R326Q polymorphisms. (A-B) Washed platelets isolated from healthy heterozygous FcγRIIA 131H/R donors (n = 20) were incubated with murine IgG1 monoclonal antibodies ALB6 (2.5 μg/mL) and PL2-49 (7.5 μg/mL). Lag time (A) and maximum aggregation (B) were analyzed according to CD148 polymorphisms (▩ noncarriers = 13 and ▨ carriers of the 276P/326Q alleles = 7). (C-D) Washed platelets isolated from healthy donors (n = 33) were incubated with 0.5, 1, or 10 μg/mL collagen or with 0.05 IU/mL thrombin. Lag time (C) and maximum aggregation (D) were analyzed in relation to CD148 polymorphisms (▩ noncarriers = 21 and ▨ carriers of the 276P/326Q alleles = 12). The horizontal lines in the middle of box-and-whiskers plots show the median values. The tops and bottoms of boxes show the 25th and 75th percentiles. The whiskers define the 10th and 90th percentiles. Mann-Whitney U test was performed to compare aggregation parameters in relation to polymorphisms of CD148, and significant P values are displayed.
We also investigated the influence of CD148 polymorphisms on platelet aggregation induced by collagen or thrombin tested as control. The lag time was significantly longer with platelets from donors heterozygous for the 276P/326Q alleles when aggregation was induced by the lowest concentrations of collagen (median = 67 vs 52 seconds with 276QQ/326RR platelets; P = .045 and 102 vs 68 seconds; P = .03 with 1 and 0.5 μg/mL collagen, respectively; Figure 2C). In addition, the maximum aggregation recorded with 0.5 μg/mL collagen was also lower (median = 49% vs 65%, P = .015; Figure 2D). In contrast, no significant difference was observed in platelet response to collagen according to the CD148 D872E genotype (supplemental Figure 1).
Collagen- and FcγRIIA-dependent phosphorylation according to CD148 polymorphisms
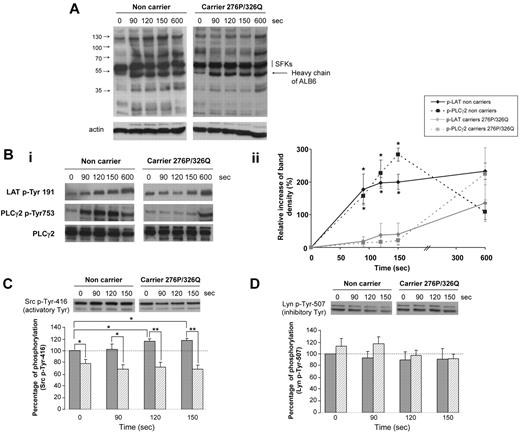
We then studied the kinetics of tyrosine phosphorylation in platelets activated by ALB6 in nonaggregating conditions to prevent αIIbβ3-dependent processes. The pattern of tyrosine phosphorylation seemed similar in resting platelets (T0) whether they were expressing the mutated form of CD148 or not (Figure 3A). However, several proteins were tyrosine-phosphorylated in nonmutated platelets after 90 seconds of activation by ALB6, whereas no significant change in the phosphorylation pattern was evidenced even after 150 seconds stimulation of platelets expressing the CD148 276P/326Q isoforms. The tyrosine phosphorylation of LAT and phospholipase Cγ2 (PLCγ2), 2 well-known signaling proteins downstream from FcγRIIA, also was significantly reduced in these platelets at the early times of stimulation, but not at 600 seconds (Figure 3B). This time course, indicating a delay in the onset of tyrosine phosphorylation, probably explains the longer lag time recorded with 276QP/326RQ heterozygous platelets before aggregation. Specific analyses also revealed lower levels of activatory Src-Tyr 416 phosphorylation in resting platelets from heterozygous donors (Figure 3C). Although this phosphorylation increased after FcγRIIA cross-linking in control platelets, no significant change could be observed in platelets with the 276P/326Q alleles. Conversely, the phosphorylation of the inhibitory Tyr 507 of Lyn seemed higher in platelets from CD148 276QP/326RQ donors compared with control homozygous cells, but the difference observed was not significant (P = .1; Figure 3D). In addition, a delay in tyrosine phosphorylation was also observed in heterozygous 276QP/326RQ platelets after stimulation with low doses of collagen (0.5 μg/mL; supplemental Figure 2).
Kinetics of phosphorylation after FcγRIIA-dependent activation. Washed platelets from carriers (n = 3, ▨) and noncarriers of the CD148 276P/326Q alleles (n = 3, ▩) were activated by 2.5 μg/mL ALB6 in the absence of aggregation (tirofoban) and then lysed at 0, 90, 120, or 150 seconds. Platelet lysates were western blotted with an anti–phospho-tyrosine and antiactin (A), or antibodies against LAT p-Tyr 191, PLCγ2 p-Tyr 753, and PLCγ2 (Bi-ii), an anti-Src p-Tyr 146 (C), or an anti-Lyn p-Tyr-507 (D). In panels Bii, C, and D, band intensities were quantified (mean ± SEM; *P < .05 and ** P < .01, t test).
Kinetics of phosphorylation after FcγRIIA-dependent activation. Washed platelets from carriers (n = 3, ▨) and noncarriers of the CD148 276P/326Q alleles (n = 3, ▩) were activated by 2.5 μg/mL ALB6 in the absence of aggregation (tirofoban) and then lysed at 0, 90, 120, or 150 seconds. Platelet lysates were western blotted with an anti–phospho-tyrosine and antiactin (A), or antibodies against LAT p-Tyr 191, PLCγ2 p-Tyr 753, and PLCγ2 (Bi-ii), an anti-Src p-Tyr 146 (C), or an anti-Lyn p-Tyr-507 (D). In panels Bii, C, and D, band intensities were quantified (mean ± SEM; *P < .05 and ** P < .01, t test).
Inhibitory effect of dasatinib on FcγRIIA-dependent platelet activation according to CD148 polymorphisms
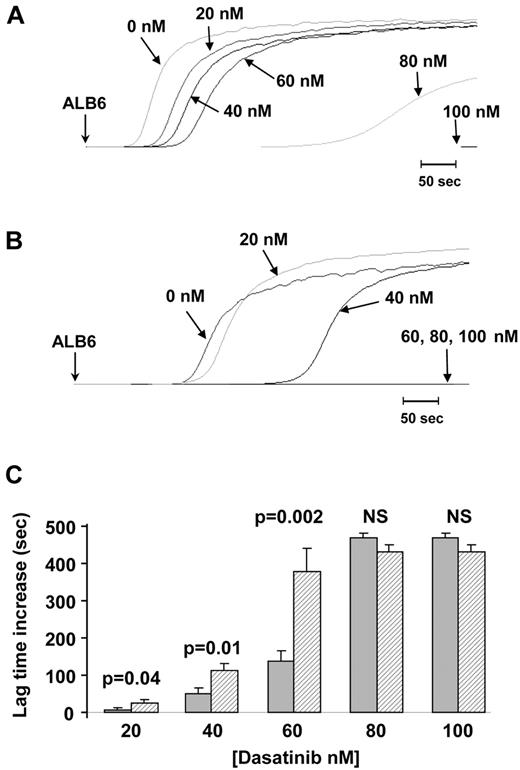
Washed platelets were preincubated with increasing concentrations of dasatinib (0, 20, 40, 60, 80, and 100nM) before activation by 1 μg/mL collagen or 2.5 μg/mL ALB6. Platelet aggregation induced by ALB6 was fully inhibited by 100nM dasatinib whatever the CD148 genotype (Figure 4). In addition, the lag time was prolonged with concentrations of dasatinib < 80nM (20, 40, and 60nM), but this effect was consistently more pronounced with platelets from heterozygous Q276P/R326Q donors (Figure 4). Similar dose-response effects in relation to CD148 polymorphisms were obtained when platelet aggregation was induced by collagen (supplemental Figure 3). These findings indicate that platelets from CD148 Q276P/R326Q donors exhibited greater sensitivity to SFK inhibitors than control platelets.
Inhibition of FcγRIIA-dependent platelet aggregation by dasatinib according to CD148 Q276P/R326Q polymorphisms. Platelets from 10 healthy donors were treated with increasing concentrations of dasatinib (0, 20, 40, 60, 80, and 100nM) before induction of aggregation by ALB6 (2.5 μg/mL). Aggregation curves (A-B) are representative of results obtained with platelets from noncarriers (A) and carriers of the CD148 276P/326Q isoforms (B). The mean increase in lag time (± SEM) in the presence of dasatinib was compared (t test) between noncarriers (n = 6, ▩) and carriers of the CD148 276P/326Q isoforms (n = 4, ▨).
Inhibition of FcγRIIA-dependent platelet aggregation by dasatinib according to CD148 Q276P/R326Q polymorphisms. Platelets from 10 healthy donors were treated with increasing concentrations of dasatinib (0, 20, 40, 60, 80, and 100nM) before induction of aggregation by ALB6 (2.5 μg/mL). Aggregation curves (A-B) are representative of results obtained with platelets from noncarriers (A) and carriers of the CD148 276P/326Q isoforms (B). The mean increase in lag time (± SEM) in the presence of dasatinib was compared (t test) between noncarriers (n = 6, ▩) and carriers of the CD148 276P/326Q isoforms (n = 4, ▨).
Discussion
The HIT immune response is characterized by a rapid IgG antibody response to PF4/Hs and is enhanced by clinical circumstances associated with platelet activation.25 However, the mechanisms that regulate the synthesis and pathogenicity of HIT antibodies have not been fully defined and are possibly influenced by genetic factors. In this study, we investigated the potential impact of 3 amino-acid variations (Q276P, R326Q, and D872E) affecting CD148 on the synthesis of antibodies to PF4/Hs and the occurrence of HIT. Our results demonstrated that patients carrying the CD148 276P and 326Q alleles seemed less at risk of HIT, although they had developed significant levels of antibodies to H/PF4 (OR = 0.47, 95% CI, 0.25%-0.89% and OR = 0.50, 95% CI, 0.27%-0.92%, respectively). In accordance with previous studies, we found a strong linkage disequilibrium between both CD148 Q276P and R326Q polymorphisms.26 Importantly, our results suggested that CD148 Q276P and R326Q polymorphisms influence the pathogenicity of PF4/H antibodies but not the immune response to heparin because the minor allele frequency was different between HIT and Abpos patients, but not between Abneg and Abpos controls.
Like CD45, CD148 is a receptor-like PTP expressed in immune cells and a positive regulator of Src family kinases in B cells and macrophages.16,17,27 These redundant roles of CD148 and CD45 could therefore explain why no association was found between the potentially functional variations of CD148 analyzed in our study and the development of antibodies to PF4/Hs. Moreover, CD148 is present in platelets in larger amounts than CD45, which is marginally expressed in these blood cells.28
HIT IgG antibodies bind to FcγRIIA, a low-affinity receptor present at low copy numbers in platelets (1000-2000 receptors/cell). However, PF4/H/IgG immune complexes may strongly activate platelets by cross-linking FcγRIIA receptors, and this process is a key event in pathogenesis of HIT.29 In our study, HIPA tests performed with HIT plasma showed that platelets isolated from healthy donors carrying the CD148 276P/326Q alleles were hyporesponsive to anti-PF4/H antibodies. In addition, subjects expressing the CD148 276P/326Q isoform exhibited a lower response to murine monoclonal antibodies ALB6 and PL2-49, which also activate platelets via FcγRIIA receptors.23,24 Moreover, these subjects were hyporesponsive to low concentrations of collagen (1 and 0.5 μg/mL), whereas platelet responses to high doses of collagen and thrombin were similar to those of nonmutated individuals. These results are consistent with those obtained previously with murine CD148-deficient platelets that failed to aggregate when activated by low doses of collagen but that responded normally to higher doses.15,30 Moreover, our experiments showed that the lag time measured after activation of platelets by HIT antibodies or monoclonal antibodies cross-linking with FcγRIIA was more influenced by CD148 polymorphisms than the maximum aggregation. Delayed FcγRIIA-dependent signaling that particularly affected LAT and PLCγ2 tyrosine phosphorylation was demonstrated in cells expressing the CD148 276P/326Q isoforms. Moreover, the phosphorylation of the activatory Tyr 416 of SFK seemed lower in platelets of CD148 276P/326Q donors, whereas the inhibitory Tyr-phosphorylation of Lyn tended to be higher. In addition, platelets expressing the 276P/326Q alleles were more sensitive to inhibition by the SFK inhibitor dasatinib than 276QQ/326RR cells. These variations in phosphorylation evidenced in human platelets in our study are compatible with previous findings obtained with CD148-deficient murine platelets, and they support the critical role of CD148 in regulating both collagen and FcγRIIA-dependent signaling pathways. Taken together, these results demonstrate that FcγRIIA- or collagen-dependent signaling pathways are less efficient in platelets exhibiting the CD148 276P/326Q polymorphism. This could be attributed to reduced activation of SFKs and suggests that the threshold of activation was higher within platelets expressing the 276P/326Q alleles. This is consistent with recent findings that demonstrated that CD148 contributes to maintaining a pool of active SFKs in resting platelets and is necessary for optimal platelet activation by collagen.30 Our results also showed that the membrane expression of CD148 was similar in platelets whatever the CD148 genotype, suggesting that the presence of proline and glutamine at positions 276 and 326, respectively, might be associated with lower phosphatase activity. The substitutions Q276P and R326Q induce torsional stress and a loss of positive charge in the second fibronectin type III (FNIII-2) domain, respectively,18 and these changes could also modify the ligand binding capacity of CD148 or its compartmentalization into membrane microdomains. Supporting this hypothesis, it has been demonstrated that the ectodomain oligomerization of 2 other receptor-like protein tyrosine phosphatases, that is, stomach cancer-associated protein tyrosine phosphatase-1 and protein tyrosine phosphatase receptor type O, is important to promote the tyrosine phosphorylation that is essential for regulating their phosphatase activity31-33 In addition, Takahashi et al showed that ectodomain-mediated oligomerization of CD148 was important for its phosphatase activity, with a critical role of 8 amino acids and particularly of residue 276Q.34
In contrast, Senis et al demonstrated that thrombus formation induced by vascular lesions was delayed in CD148 transmembrane-deficient mice.15 In our study, no association could be found between the CD148 polymorphisms analyzed and thrombotic events in HIT patients (data not shown), but the number of samples evaluated was relatively small. Moreover, other cell types including monocytes and endothelial cells also can be activated by HIT antibodies and might have an important role in the development of thrombosis in HIT.35-37
In conclusion, our findings support the hypothesis that CD148 constitutes an important regulatory element for both collagen and FcγRIIA-mediated human platelet activation. In addition, they demonstrate that a single polymorphism of this signaling protein can affect platelet reactivity. In addition with individual variations in platelet signaling pathways, differences in the epitope specificity or binding properties of HIT antibodies (affinity) also might contribute to their pathogenicity as supported by 2 different studies performed with human38 or murine39 antibodies to PF4/Hs. FcγRIIA has recently been shown to have a critical role in platelet thrombus formation40 and our study therefore also suggests that analysis of CD148 P276Q and Q326R polymorphisms might contribute to evaluation of the variability of platelet responses in various diseases associated with thrombosis, including HIT.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Hsueh Cheng Sung for help with flow cytometry analysis and Doreen Raine for editing.
This study was supported by the Institut pour la Recherche sur la Thrombose et l'Hémostase.
Authorship
Contribution: J.R. performed and designed the research, analyzed the data, and wrote the paper; C.P. and Y.G. designed the research, analyzed the data, and wrote the paper; M.-P.G. performed research and analyzed the data; D.L. and V.G.-G. performed experiments; M.A.M. and M.A. contributed to the collection of samples; and B.P. analyzed the data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yves Gruel, Department of Hematology-Hemostasis, CHU Trousseau, 37044 Tours Cedex, France; e-mail: gruel@med.univ-tours.fr.