Abstract
Platelet adhesion and aggregation play a critical role in primary hemostasis. Uncontrolled platelet activation leads to pathologic thrombus formation and organ failure. The decisive central step for different processes of platelet activation is the increase in cytosolic Ca2+ activity ([Ca2+]i). Activation-dependent depletion of intracellular Ca2+ stores triggers Ca2+ entry from the extracellular space. Stromal interaction molecule 1 (STIM1) has been identified as a Ca2+ sensor that regulates store-operated Ca2+ entry through activation of the pore-forming subunit Orai1, the major store-operated Ca2+ entry channel in platelets. In the present study, we show for the first time that the chaperone protein cyclophilin A (CyPA) acts as a Ca2+ modulator in platelets. CyPA deficiency strongly blunted activation-induced Ca2+ mobilization from intracellular stores and Ca2+ influx from the extracellular compartment and thus impaired platelet activation substantially. Furthermore, the phosphorylation of the Ca2+ sensor STIM1 was abrogated upon CyPA deficiency, as shown by immunoprecipitation studies. In a mouse model of arterial thrombosis, CyPA-deficient mice were protected against arterial thrombosis, whereas bleeding time was not affected. The results of the present study identified CyPA as an important Ca2+ regulator in platelets, a critical mechanism for arterial thrombosis.
Introduction
Platelet adhesion and aggregation are essential for hemostasis at sites of vascular injury to avoid excessive blood loss.1,2 In contrast, uncontrolled platelet activation can induce acute vessel occlusion, leading to myocardial infarction or stroke at areas of atherosclerotic plaque rupture.3,4 Platelet activation and thrombus formation is a multistage process that involves different signaling pathways to trigger platelet shape change, integrin activation, and degranulation.4,5 The signaling pathways converge in the activation of phospholipase C (PLC), leading to the formation of inositol 1,4,5-triphosphate (IP3) and diacylglycerol.6 IP3 is then able to bind to its receptor at the endoplasmic reticulum (ER) and mediates Ca2+ efflux from the intracellular stores into the cytoplasm.6,7 Compromised PLC activation impairs Ca2+ mobilization, which is followed by defective thrombus formation under flow conditions.8,9
Stromal interaction molecule 1 (STIM1) is a type I single-transmembrane protein with an N-terminal EF hand domain (helix-loop-helix structural domain) that binds Ca2+ in the lumen of the ER. STIM1 binding to Ca2+ is interrupted upon store release and induces redistribution of STIM1 to the plasma membrane to open store-operated Ca2+ (SOC) channels, thereby stimulating store-operated Ca2+ entry (SOCE).10,11 Orai1 was identified as the major SOCE channel in platelets known to be regulated by the Ca2+ sensor STIM1.12,13
Immunophilins are endogenous cytosolic peptidyl-prolyl isomerases (PPIs) that interconvert between the cis and trans positions of target proteins.14,15 According to their sensitivity to immunosuppressor drugs, they have been classified into cyclophilins (CyPs), which form a complex with cyclosporin A (CsA), and immunophilins, which are sensitive to tacrolimus.16,17 Immunophilins are known to participate in Ca2+ homeostasis in different cells and are known to control Ca2+ channels in the ER-like IP3 receptors, whereas cyclophilins such as CyPA are probably involved in the regulation of sarcoplasmic/endoplasmic reticulum Ca2+ ATPase 2b (SERCA2b).18 CyPA is a ubiquitously expressed intracellular protein with a peptidyl-prolyl cis-trans isomerase activity and acts as a chaperone protein.14-16 It is the major cellular target of the immunosuppressive drug cyclosporine and forms a complex with calcineurin to prevent calcineurin from regulating cytokine gene transcription.17 Under inflammatory conditions, CyPA is released from immune cells and binds to its receptor, EMMPRIN (CD147), to modulate signal transduction of the target cell.19-24 Recent studies provide evidence that CyPA is also present in the cytoplasm of platelets and can be released from granules upon platelet activation to bind to endothelial cells, monocytes, and macrophages via the CyPA receptor EMMPRIN (CD147), thereby inducing a signaling cascade into the target cell (eg, degranulation or expression of matrix metalloproteinases.19,23,25-28 The function of intracellular CyPA in platelets has not yet been explored.
In the present study, we show for the first time that CyPA deficiency leads to multiple platelet activation defects caused by strongly reduced Ca2+ mobilization from intracellular stores and Ca2+ entry from the extracellular space. Our results identify CyPA as a powerful Ca2+ regulator in platelets.
Methods
Chemicals and Abs
Platelets were activated by ADP (Sigma-Aldrich), thrombin (Roche Diagnostics), convulxin (CVX; Enzo Life Sciences), and collagen-related peptide (CRP; from Richard Farndale, University of Cambridge, Cambridge, United Kingdom). Fluorophore-labeled Abs anti–P-selectin-FITC (Wug.E9-FITC, rat IgG2b; Emfret Analytics) and anti-integrin αIIbβ3-PE (JON/A-PE, rat IgG1; Emfret Analytics) were used for flow cytometric analysis. Anti-STIM1 Ab (Abnova), anti-Orai1 Ab (Santa Cruz Biotechnology), anti–α-tubulin Ab (Cell Signaling Technology), anti-CyPA Ab (Abcam), anti-SERCA2b Ab, and ERK1/2 Abs (Cell Signaling Technology) were used for Western blot analysis and immunoprecipitation studies.
Mice
Gene-targeted mice lacking CyPA (Cypa−/−) and their corresponding wild-type littermates (Cypa+/+) were bred from breeder pairs bought from The Jackson Laboratory (129.Cg-Ppiatm1Lubn/J) as described previously.28 Animal studies were approved by the local authorities (regional board Tübingen) and conducted according to German law for the welfare of animals.
Platelet preparation
Platelets were prepared as described previously.8,28-30 Briefly, murine blood from the retroorbital plexus was collected and centrifuged at 640g for 5 minutes at room temperature. To obtain platelet-rich plasma, the supernatant was centrifuged at 260g for 6 minutes. Platelet-rich plasma was washed twice at 1000g for 5 minutes at room temperature and the pellet resuspended in Tyrode buffer (136mM NaCl, 0.4mM Na2HPO4, 2.7mM KCl, 12mM NaHCO3, 0.1% glucose, and 0.35% BSA, pH 7.4) supplemented with prostacyclin (0.5μM) and apyrase (0.02 U/mL).31 Before use, platelets were resuspended in the same buffer and incubated at 37°C for 30 minutes.
Flow cytometry
Flow cytometric analysis was performed as described previously.9,32 Briefly, 2-color analysis of murine platelet activation was performed using fluorophore-labeled Abs for P-selectin expression (Wug.E9-FITC) and the active form of αIIbβ3 integrin (JON/A-PE). Heparinized blood from Cypa+/+ and Cypa−/− mice was diluted in Tyrode buffer and washed twice. Blood samples were mixed with Abs after the addition of 1mM CaCl2 and stimulated with the indicated agonists for 15 minutes at room temperature. The reaction was stopped by the addition of PBS and samples were analyzed on a FACSCalibur flow cytometer (BD Biosciences).
Measurement of ATP release
The determination of ATP release after platelet stimulation was performed as described previously.9 Briefly, platelets were activated with the indicated agonists and fixed for 2 hours. ATP levels were quantified using the bioluminescence assay kit from Roche Diagnostics.
Spreading experiments
Spreading experiments were performed as described previously.8 Briefly, coverslips (24 × 60 mm) were coated with human fibrinogen (1 mg/mL) overnight and blocked for 1 hour with 1% BSA in PBS. Washed platelets from Cypa+/+ and Cypa−/− mice were resuspended to a final concentration of 1 × 105 platelets/μL, seeded on the coverslip, and incubated at room temperature for the indicated time points. To achieve full platelet spreading on fibrinogen, platelets were activated with 0.008 U/mL of thrombin before seeding. After incubation, unbound platelets were removed by rinsing with Tyrode buffer and adherent platelets were fixed with 4% paraformaldehyde and stained for F-actin using rhodamine phalloidin. At least 8 confocal images were taken for each sample and analyzed for adhesion, filopodia, and lamellipodia formation (fully spread platelets).
Flow chamber
Coverslips (24 × 60 mm) were coated with 200 μg/mL of fibrillar type I collagen (Nycomed) overnight and then blocked with 300 μL of 1% BSA solution for at least 30 minutes. Tyrode buffer was prepared as described previously.9 The pH was adjusted to 7.4 and the Tyrode buffer was prewarmed at 37°C. Mice were anesthetized with isoflurane and 700 μL of blood was taken from the retroorbital plexus of each mouse and collected in a tube containing 300 μL of heparin (20 U/mL in TBS). Two parts of blood mixed with 1 part of Tyrode buffer were incubated at 37°C and put in a 1-mL syringe. The desired shear stress of 1000/s or 1700/s was adjusted and thrombus formation was measured as the percentage of area covered by thrombi within a certain area.
Measurement of IP1
For the determination of IP1 concentration after platelet stimulation, the IP-One ELISA kit from Cisbio was used and experiments were performed as described previously.9
Intracellular Ca2+ measurements
Ca2+ measurements were performed as described previously.8,9 Briefly, washed platelets from Cypa+/+ and Cypa−/− mice were resuspended in Tyrode buffer without Ca2+ and loaded with 5μM Fura-2 acetoxymethyl ester (Invitrogen) and 0.2 μg/mL of Pluronic F-127 (Biotium) at 37°C for 30 minutes. Platelets were washed once and resuspended in Tyrode buffer containing 0.5mM EGTA (Roth) and 1mM Ca2+, respectively. After platelet activation with different agonists, the Ca2+ response was measured under stirring conditions using a spectrofluorometer (LS 55; PerkinElmer) at alternate excitation wavelengths of 340 and 380 nm. The ratio values of 340/380 nm were converted into Ca2+ concentrations (Ca2+) by lysis of platelets with Triton X-100 (Sigma-Aldrich) and EGTA. Measurements with thapsigargin (TG; Invitrogen) were performed in Ca2+-free Tyrode buffer. Incubation of platelets with 5μM TG for 10 minutes was followed by the addition of 1mM Ca2+ to measure Ca2+ influx in Cypa+/+ and Cypa−/− platelets.
Cell culture and transfections
A5-CHO cells were cultured in DMEM at 37°C in a 5% CO2 atmosphere. For transient protein expression of green fluorescent protein (GFP)–tagged STIM1, cells were seeded in 6-well cell-culture plates 24 hours before transfection. Transfection was carried out using 4 μg of plasmid DNA and 10 μL of Lipofectamine 2000 per dish.
Spreading and immunohistology
A5-CHO cells were seeded on coverslips and incubated at room temperature for 60 minutes. Adherent cells were fixed and permeabilized. After washing with PBS, the slides were stained and fluorescence was detected using a confocal laser scanning microscope.
Bleeding time
Mice were anesthetized with medetomidine, midazolam, and fentanyl and a 1-mm piece of the tail was cut off with a scalpel. Blood drops were collected every 20 seconds on a filter paper without touching the lesion until blood flow stopped. Experiments were stopped after 20 minutes using tissue glue if no stop of blood flow occurred.
Intravital microscopy of thrombus formation in mesenteric arterioles injured with FeCl3
Mice (5-6 weeks of age) mice were anesthetized with medetomidine, midazolam, and fentanyl. After a midline abdominal incision, the mesentery was exteriorized and arterioles free of fat tissue were injured by topical application of a filter paper saturated with 20% FeCl3 for 10 seconds.
Thrombus formation was made visible by IV injection of rhodamine and observed with a fluorescence microscope. Time until full occlusion of the vessel (ie, when blood flow had stopped for > 1 minute) was measured. Experiments were stopped after 40 minutes.
Data analysis
Data are given as means ± SD from at least 3 individual experiments (n represents the number of experiments). All data were tested for significance using the paired or unpaired Student t test and the Mann-Whitney U test. P < .05 was considered statistically significant.
Results
CyPA deficiency leads to reduced β3-integrin activation and degranulation, delayed spreading, and defective thrombus formation under flow
To investigate the role of CyPA in platelets, we compared wild-type (Cypa+/+) and CyPA-deficient (Cypa−/−) mice. CyPA deficiency in platelets was confirmed by Western blot (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Platelet count and size were not different between Cypa+/+ and Cypa−/− mice, indicating that CyPA is not important for platelet generation from megakaryocytes of the BM (supplemental Figure 1B). To determine the relevance of CyPA for platelet activation, degranulation (P-selectin expression) and β3-integrin activation (JON/A-binding) in response to different agonists were analyzed by flow cytometry. Whereas β3-integrin activation in response to ITAM-coupled receptors (eg, GPVI) was not changed, a strong reduction was measured after G-protein–coupled receptor activation (eg, PAR or P2Y12) using intermediate concentrations of thrombin and ADP (Figure 1A). In addition, the exposure of P-selectin, which is used as a marker for α-granule release, was also strongly reduced after thrombin and ADP stimulation, but not in response to CRP and CVX (Figure 1B). The defective degranulation of CypA-deficient platelets was also shown by a decrease in thrombin-induced release of ATP, demonstrating that degranulation per se was defective after G-protein–coupled receptor activation (Figure 1E). To determine the impact of CyPA on cytoskeletal reorganization, Cypa+/+ and Cypa−/− platelets were allowed to adhere and spread on a fibrinogen matrix using thrombin stimulation to induce full spreading of murine platelets.33 Spreading of platelets was analyzed by the development of filopodia and lamellipodia at different time points. Subsequently, platelets were stained with rhodamine phalloidin and analyzed by confocal microscopy (Figure 1C). Surprisingly, spreading of Cypa−/− platelets was delayed significantly. After 20 minutes, only 22.3% ± 8.5% of Cypa−/− platelets show filopodia formation versus 39.7% ± 3.9% for Cypa+/+ platelets (P < .001), whereas 22.2% ± 5.5% of Cypa+/+ platelets were already fully spread (Cypa−/− platelets, 2.2% ± 2.1%; P < .001; Figure 1D). However, after 60 minutes, most of the CyPA-deficient platelets were able to fully spread indicating that spreading per se was not impaired (48.2% ± 4.5% of Cypa+/+ platelets vs 55.2% ± 2.6% of Cypa−/− platelets; P = .285; Figure 1C-D).
CyPA deficiency leads to reduced αIIbβ3-integrin activation, degranulation, delayed spreading, and defective thrombus formation under flow. (A) Blood from Cypa+/+ and Cypa−/− mice was washed twice and incubated with 1 and 5 μg/mL of CRP, 0.002 and 0.02 U/mL of thrombin, 2 and 10μM ADP, and 1 μg/mL of CVX for 15 minutes in the presence of JON/A-PE, which only binds to the activated form of integrin αIIbβ3. Platelets were gated by their forward and side scatter characteristics. For each measurement, the mean fluorescence intensity (MFI) is shown for JON/A-PE (A) and anti–P-selectin-FITC (B; n = 6 per group). *P < .05; **P < .01; ***P < .001. (C) Platelets from the indicated mice were washed and allowed to spread on immobilized human fibrinogen (1 mg/mL) for 20 and 60 minutes after thrombin stimulation. Representative confocal images of 6 individual experiments are shown. Scale bar indicates 10 μm. (D) Quantitative assessment of adherent platelets, filopodia formation, and the number of fully spread platelets at different time points (20 and 60 minutes). Bar graphs depict mean values ± SD (n ≥ 6 mice per group). After 20 minutes, fully spread platelets were 22.3% ± 5.5% of Cypa+/+ platelets versus 2.2% ± 2.1% of Cypa−/− platelets; filopodia formation, 39.7% ± 3.9% of Cypa+/+ platelets versus 22.3% ± 8.6% of Cypa−/− platelets; platelet adhesion, 38% ± 5.5% of Cypa+/+ platelets versus 75.5% ± 8.1% of Cypa−/− platelets. After 60 minutes, fully spread platelets were 48.2% ± 4.5% of Cypa+/+ platelets versus 55.2% ± 2.6% of Cypa−/− platelets; filopodia formation, 39.5% ± 2.5% of Cypa+/+ platelets versus 36.2% ± 2.5% of Cypa−/− platelets; platelet adhesion, 12.3% ± 3.8% of Cypa+/+ platelets versus 8.7% ± 1.51% of Cypa−/− platelets. (E) Release of ATP on platelet stimulation with indicated agonists. Determination of ATP in the supernatant of platelets using a luminometric assay. Results are shown as the mean micromolar ATP concentration ± SD (n = 4 per group). Thr indicates thrombin. (F) Thrombus formation under flow. Whole blood was perfused over a collagen matrix at the indicated shear rates. Bar graphs depict mean values of thrombus surface coverage at a shear rate of 1000/s and 1700/s ± SD (n = 8 per group). U46619 (3μM) was added to whole blood before it entered the flow chamber as indicated. (G) Representative phase-contrast images at a shear rate of 1000/s (left panel) and 1700/s (right panel) are shown. Scale bar indicates 50 μm.
CyPA deficiency leads to reduced αIIbβ3-integrin activation, degranulation, delayed spreading, and defective thrombus formation under flow. (A) Blood from Cypa+/+ and Cypa−/− mice was washed twice and incubated with 1 and 5 μg/mL of CRP, 0.002 and 0.02 U/mL of thrombin, 2 and 10μM ADP, and 1 μg/mL of CVX for 15 minutes in the presence of JON/A-PE, which only binds to the activated form of integrin αIIbβ3. Platelets were gated by their forward and side scatter characteristics. For each measurement, the mean fluorescence intensity (MFI) is shown for JON/A-PE (A) and anti–P-selectin-FITC (B; n = 6 per group). *P < .05; **P < .01; ***P < .001. (C) Platelets from the indicated mice were washed and allowed to spread on immobilized human fibrinogen (1 mg/mL) for 20 and 60 minutes after thrombin stimulation. Representative confocal images of 6 individual experiments are shown. Scale bar indicates 10 μm. (D) Quantitative assessment of adherent platelets, filopodia formation, and the number of fully spread platelets at different time points (20 and 60 minutes). Bar graphs depict mean values ± SD (n ≥ 6 mice per group). After 20 minutes, fully spread platelets were 22.3% ± 5.5% of Cypa+/+ platelets versus 2.2% ± 2.1% of Cypa−/− platelets; filopodia formation, 39.7% ± 3.9% of Cypa+/+ platelets versus 22.3% ± 8.6% of Cypa−/− platelets; platelet adhesion, 38% ± 5.5% of Cypa+/+ platelets versus 75.5% ± 8.1% of Cypa−/− platelets. After 60 minutes, fully spread platelets were 48.2% ± 4.5% of Cypa+/+ platelets versus 55.2% ± 2.6% of Cypa−/− platelets; filopodia formation, 39.5% ± 2.5% of Cypa+/+ platelets versus 36.2% ± 2.5% of Cypa−/− platelets; platelet adhesion, 12.3% ± 3.8% of Cypa+/+ platelets versus 8.7% ± 1.51% of Cypa−/− platelets. (E) Release of ATP on platelet stimulation with indicated agonists. Determination of ATP in the supernatant of platelets using a luminometric assay. Results are shown as the mean micromolar ATP concentration ± SD (n = 4 per group). Thr indicates thrombin. (F) Thrombus formation under flow. Whole blood was perfused over a collagen matrix at the indicated shear rates. Bar graphs depict mean values of thrombus surface coverage at a shear rate of 1000/s and 1700/s ± SD (n = 8 per group). U46619 (3μM) was added to whole blood before it entered the flow chamber as indicated. (G) Representative phase-contrast images at a shear rate of 1000/s (left panel) and 1700/s (right panel) are shown. Scale bar indicates 50 μm.
To elucidate the relevance of CyPA for platelet adhesion and thrombus formation under flow conditions, we perfused whole blood over a collagen-coated surface. At all shear rates tested (ie, 1000/s and 1700/s), both Cypa+/+ and Cypa−/− platelets adhered to the collagen matrix and formed aggregates (Figure 1G). However, whereas Cypa+/+ platelets were able to form massive thrombi, small aggregates of Cypa−/− platelets were unstable and frequently washed away, thereby abrogating thrombus formation. In this case, the collagen matrix was covered by a single platelet layer and small aggregates of Cypa−/− platelets, demonstrating strongly reduced thrombus surface coverage independent of shear rates tested (Figure 1F). Defective thrombus formation was also observed after CsA treatment of platelets (supplemental Figure 2), suggesting that the enzymatic activity of CyPA is responsible for this defect.
To determine whether thrombus formation of CyPA-deficient platelets can be recovered by the addition of secondary agonists such as TxA2, we coinfused U46619 and CyPA-deficient blood directly before it entered the flow chamber (Figure 1F-G).9 Indeed, TxA2-mediated platelet activation led to stable thrombus formation of CyPA-deficient platelets, suggesting that appropriate stimulation of these platelets can induce the formation of stable 3-dimensional thrombi under flow.
Multiple platelet activation defects in CyPA-deficient platelets are caused by defective Ca2+ mobilization
Different processes of platelet activation, including integrin activation, degranulation, cytoskeletal reorganization, and thrombus formation, are depending on proper Ca2+ mobilization. Therefore, spectrofluorometric measurements were performed to analyze the impact of CyPA on the increase of cytosolic Ca2+ after platelet activation using different agonists to stimulate G-protein–coupled and ITAM-coupled signaling pathways. Experiments were done in the presence (1mM Ca2+) and absence (0.5mM EGTA) of extracellular Ca2+ to distinguish between store release and influx of extracellular Ca2+. Before stimulation, the intracellular Ca2+ concentration was comparable between Cypa+/+and Cypa−/− platelets (Figure 2A). Agonist stimulation triggered a Ca2+ influx from extracellular space in Cypa+/+and Cypa−/− platelets. However, the increase of cytosolic Ca2+ was less pronounced in Cypa−/− platelets (Figure 2A-B left panel), suggesting a role of CyPA in the regulation of SOCE. Furthermore, measurements of agonist-induced changes in [Ca2+]i revealed a clear reduction of store release in response to thrombin and CRP (Figure 2A-B right panel). These defects in intracellular, as well as the extracellular Ca2+ mobilization in Cypa−/− platelets, suggested a general defect in Ca2+ homeostasis induced by CyPA deficiency. To test this hypothesis, we treated Fura-2–loaded platelets with the SERCA inhibitor TG.11 In the absence of extracellular Ca2+, TG (5μM) triggered a Ca2+ release from intracellular stores that was reduced significantly in Cypa−/− platelets compared with Cypa+/+ platelets (Figure 2C). The subsequent addition of extracellular Ca2+ induced SOCE that was also reduced strongly in Cypa−/− platelets compared with Cypa+/+ platelets (Figure 2C). These results demonstrate for the first time that CyPA is involved in store release and SOCE, indicating a role for CyPA as a powerful Ca2+ regulator in platelets.
Intracellular CyPA regulates Ca2+ mobilization in platelets. (A-B) Defective agonist-induced Ca2+ mobilization and impaired SOCE in Cypa+/+ and Cypa−/− platelets. Fura-2–loaded Cypa+/+ (black line) and Cypa−/− (gray line) platelets before and after stimulation with thrombin (0.02 U/mL) or CRP (10 μg/mL) in the absence (0.5mM EGTA, right) or presence (1mM Ca2+, left) of extracellular Ca2+. (A) Representative tracings reflecting cytosolic Ca2+ concentration ([Ca2+]i). (B) Arithmetic mean of maximal increase in intracellular Ca2+ concentrations compared with baseline levels before stimulus (Δ [Ca2+]i ± SD, n ≥ 6 per group). *P < .05; **P < .01; ***P < .001. (C) Fura-2 fluorescence reflecting cytosolic Ca2+ concentration ([Ca2+]i) of Cypa+/+ (black line) and Cypa−/− platelets (gray line) after stimulation with 5μM thapsigargin for 10 minutes, followed by the addition of 1mM extracellular Ca2+. Representative measurements (top panel) and Δ [Ca2+]i ± SD (n ≥ 6 per group) before and after the addition of 1mM Ca2+ (bottom panel) are presented. *P < .05; ***P < .001.
Intracellular CyPA regulates Ca2+ mobilization in platelets. (A-B) Defective agonist-induced Ca2+ mobilization and impaired SOCE in Cypa+/+ and Cypa−/− platelets. Fura-2–loaded Cypa+/+ (black line) and Cypa−/− (gray line) platelets before and after stimulation with thrombin (0.02 U/mL) or CRP (10 μg/mL) in the absence (0.5mM EGTA, right) or presence (1mM Ca2+, left) of extracellular Ca2+. (A) Representative tracings reflecting cytosolic Ca2+ concentration ([Ca2+]i). (B) Arithmetic mean of maximal increase in intracellular Ca2+ concentrations compared with baseline levels before stimulus (Δ [Ca2+]i ± SD, n ≥ 6 per group). *P < .05; **P < .01; ***P < .001. (C) Fura-2 fluorescence reflecting cytosolic Ca2+ concentration ([Ca2+]i) of Cypa+/+ (black line) and Cypa−/− platelets (gray line) after stimulation with 5μM thapsigargin for 10 minutes, followed by the addition of 1mM extracellular Ca2+. Representative measurements (top panel) and Δ [Ca2+]i ± SD (n ≥ 6 per group) before and after the addition of 1mM Ca2+ (bottom panel) are presented. *P < .05; ***P < .001.
To determine whether the activity of PLC is affected by the absence of CyPA, we analyzed IP1 production. As shown in supplemental Figure 3, thrombin- and CRP-induced IP1 production was comparable in CyPA-deficient and control platelets, demonstrating that reduced Ca2+ mobilization is not a result of altered PLCγ or PLCβ activity in those platelets (supplemental Figure 3).
Enzymatic activity of CyPA is responsible for defective Ca2+ mobilization
Cyclophilins play a role in protein folding mediated by their PPI domain, which catalyzes the isomerization of X-proline peptide bonds,34 and via their chaperone activity.35-37 Binding of CsA to CyPA leads to inhibition of the Ca2+- and calmodulin-dependent phosphatase calcineurin and inhibits the PPIase and chaperone activity of CyPA.14 To determine whether the enzymatic activity of CyPA is responsible for the defects in Ca2+ mobilization of platelets, spectrofluorometric measurements of Fura-2–loaded Cypa+/+ platelets were performed in the presence and absence of CsA. Agonist stimulation leads to a dramatic defect in store release and SOCE in CsA-treated platelets that was comparable to the results observed in Cypa−/− platelets (Figure 3A). Furthermore, the TG-induced Ca2+ mobilization from internal stores and Ca2+ entry from the extracellular space were both blunted by CsA (Figure 3B-C). These results suggest that the enzymatic activity of CyPA is responsible for the defective Ca2+ mobilization in Cypa−/− platelets.
Enzymatic activity of CyPA is responsible for defective Ca2+ mobilization. Defective agonist-induced Ca2+ mobilization and impaired SOCE in Cypa+/+ platelets pretreated with 1 μg/mL of CsA. (A) Maximal increase in cytosolic Ca2+ concentration of Cypa+/+ (black bars) and CsA-treated Cypa+/+ platelets (gray bars) after stimulation with thrombin (0.02 U/mL) and CRP (10 μg/mL), respectively. Arithmetic means of maximal [Ca2+]i ± SD (n ≥ 6 per group) before (left panel) and after (right panel) the addition of 1mM Ca2+. **P < .01; ***P < .001. (B) Representative time course of intracellular Ca2+ mobilization in Cypa+/+ (black line) and CsA-treated Cypa+/+ platelets (gray line) after exposure to 5μM thapsigargin for 10 minutes and subsequent addition of 1mM extracellular Ca2+. (C) Mean values of maximal Δ [Ca2+]i ± SD (n ≥ 5 per group) before and after the addition of 1mM Ca2+. **P < .01; ***P < .001.
Enzymatic activity of CyPA is responsible for defective Ca2+ mobilization. Defective agonist-induced Ca2+ mobilization and impaired SOCE in Cypa+/+ platelets pretreated with 1 μg/mL of CsA. (A) Maximal increase in cytosolic Ca2+ concentration of Cypa+/+ (black bars) and CsA-treated Cypa+/+ platelets (gray bars) after stimulation with thrombin (0.02 U/mL) and CRP (10 μg/mL), respectively. Arithmetic means of maximal [Ca2+]i ± SD (n ≥ 6 per group) before (left panel) and after (right panel) the addition of 1mM Ca2+. **P < .01; ***P < .001. (B) Representative time course of intracellular Ca2+ mobilization in Cypa+/+ (black line) and CsA-treated Cypa+/+ platelets (gray line) after exposure to 5μM thapsigargin for 10 minutes and subsequent addition of 1mM extracellular Ca2+. (C) Mean values of maximal Δ [Ca2+]i ± SD (n ≥ 5 per group) before and after the addition of 1mM Ca2+. **P < .01; ***P < .001.
Lack of CyPA prevents phosphorylation of STIM1
STIM1 and Orai1 are the key players in calcium signaling in platelets.10,13,46 STIM1 deficiency reduced Ca2+ efflux from intracellular stores and SOCE in a manner similar to that observed in Cypa−/− platelets.11 It is known that the inhibition of PPIase and chaperone activity of cyclophilins by CsA is able to impair protein maturation and folding followed by reduced level of functional expression.38 To ensure that impaired Ca2+ mobilization in CyPA-deficient platelets is not caused by reduced expression level of either STIM1 or Orai1 as observed in sgk1−/− mice,46 we confirmed unaltered expression levels of STIM1 and Orai1 in CyPA-deficient platelets (supplemental Figure 4). To determine whether the enzymatic activity of CyPA has any regulatory role in STIM1 activity, we analyzed colocalization of STIM1 and CyPA by immunoprecipitation experiments. As shown in Figure 4A, CyPA was found to be associated with STIM1 in Cypa+/+ platelets under resting conditions. After activation, colocalization of STIM1 and CyPA was reduced significantly, suggesting that STIM1 was released from the complex. In mutant platelets, complex formation of STIM1 and CyPA could not take place because of CyPA deficiency, and therefore, no CyPA-mediated regulation of STIM1 was possible in Cypa−/− platelets. Colocalization of CyPA and STIM1 in control and CsA-treated human platelets has been demonstrated previously by Rosado et al, who demonstrated that enzymatic activity of CyPA was necessary to release STIM1 from the complex.18 To determine the functional consequences of absent STIM1/CyPA coupling in Cypa−/− platelets, we performed phosphorylation studies to analyze STIM1 phosphorylation upon platelet activation. Immunoprecipitation experiments showed an increase in tyrosine phosphorylation of STIM1 in control platelets after thrombin stimulation for 1 and 5 minutes (Figure 4B). In contrast, no tyrosine phosphorylation of STIM1 was found in Cypa−/− platelets. Further immunoprecipitation experiments using Cypa+/+ platelets and CsA revealed that the enzymatic activity of CyPA was essential for STIM1 phosphorylation, because phosphorylation was inhibited in CsA-treated platelets, which was comparable to the results in Cypa−/− platelets (Figure 4B). These findings provide strong evidence that the PPIase activity of CyPA is important for STIM1 phosphorylation. In addition, immunoprecipitation experiments showed normal tyrosine phosphorylation of the STIM1 kinase ERK1/2 in CyPA-deficient platelets and on CsA treatment, respectively (Figure 4D).43 According to immunoprecipitation studies, the association of STIM1 and Orai1 on platelet activation was not abrogated in the mutant platelets or after CsA-mediated inhibition of CyPA (Figure 4C), suggesting that phosphorylation of STIM1 is not a prerequisite of STIM1/Orai1 coupling.
Lack of enzymatic activity of CyPA prevents phosphorylation of STIM1 and reduces cell adhesion on fibrinogen. Resting or thrombin-activated (0.1 U/mL) platelets were lysed, immunoprecipitated with the appropriate Abs, and immunoblotted with the indicated Abs detecting STIM1, Orai1, and CyPA, respectively. (A) Immunoprecipitation of whole-cell lysates of Cypa+/+ and Cypa−/− platelets with STIM1 Ab, followed by the detection of CyPA by Western blot analysis. Reproving of blots after stripping was performed with STIM1 Ab serving as a loading control. (B) Thrombin (0.1 U/mL)–stimulated Cypa+/+, Cypa−/−, and CsA-treated Cypa+/+ platelets (1 and 5 minutes) were lysed and immunoprecipitated with anti-phosphotyrosine Ab (4G10). Immunoprecipitates were analyzed by Western blot using STIM1 Ab showing rapid phosphorylation of STIM1 only in Cypa+/+ platelets. (C) Whole-cell lysates of thrombin-stimulated platelets were immunoprecipitated with Orai1 Ab, followed by Western blot analysis with STIM1 Ab showing colocalization of STIM1 and Orai1 independent of STIM1 phosphorylation. STIM1/Orai1 complex formation was also observed on CsA treatment. (D) Thrombin (0.1 U/mL)–stimulated Cypa+/+, Cypa−/−, and CsA-treated Cypa+/+ platelets (1 and 5 minutes) were lysed and immunoprecipitated with anti-phosphotyrosine Ab (4G10). Immunoprecipitates were analyzed by Western blot using ERK1/2 Ab showing comparable phosphorylation of ERK1/2 in control and Cypa−/−- and CsA-treated platelets. (E) Immunoprecipitation of platelet lysates of Cypa+/+ and Cypa−/− platelets with SERCA2b Ab, followed by the detection of CyPA by Western blot analysis. To assess that a similar amount of proteins were loaded, membranes were reproved with SERCA2b Ab serving as a loading control. (F) After thrombin stimulation, platelets were lysed and immunoprecipitated with STIM1 Ab, followed by Western blot analysis with SERCA2b Ab showing colocalization of STIM1 and SERCA2b in murine platelets. STIM1/SERCA2b complex formation was also observed in CyPA-deficient platelets and on CsA treatment. (G) A5-CHO cells expressing GFP, STIM1-GFP, or STIM1S575A/S608A/S621A-GFP were allowed to adhere and spread on human fibrinogen (1 mg/mL) for 60 minutes. STIM1 was detected by the fluorescence properties of GFP. Scale bar indicates 20 μm. (H) Statistical analysis of adhesive cells per visual field. The bar graph depicts mean values ± SD (n = 10). ***P < .001.
Lack of enzymatic activity of CyPA prevents phosphorylation of STIM1 and reduces cell adhesion on fibrinogen. Resting or thrombin-activated (0.1 U/mL) platelets were lysed, immunoprecipitated with the appropriate Abs, and immunoblotted with the indicated Abs detecting STIM1, Orai1, and CyPA, respectively. (A) Immunoprecipitation of whole-cell lysates of Cypa+/+ and Cypa−/− platelets with STIM1 Ab, followed by the detection of CyPA by Western blot analysis. Reproving of blots after stripping was performed with STIM1 Ab serving as a loading control. (B) Thrombin (0.1 U/mL)–stimulated Cypa+/+, Cypa−/−, and CsA-treated Cypa+/+ platelets (1 and 5 minutes) were lysed and immunoprecipitated with anti-phosphotyrosine Ab (4G10). Immunoprecipitates were analyzed by Western blot using STIM1 Ab showing rapid phosphorylation of STIM1 only in Cypa+/+ platelets. (C) Whole-cell lysates of thrombin-stimulated platelets were immunoprecipitated with Orai1 Ab, followed by Western blot analysis with STIM1 Ab showing colocalization of STIM1 and Orai1 independent of STIM1 phosphorylation. STIM1/Orai1 complex formation was also observed on CsA treatment. (D) Thrombin (0.1 U/mL)–stimulated Cypa+/+, Cypa−/−, and CsA-treated Cypa+/+ platelets (1 and 5 minutes) were lysed and immunoprecipitated with anti-phosphotyrosine Ab (4G10). Immunoprecipitates were analyzed by Western blot using ERK1/2 Ab showing comparable phosphorylation of ERK1/2 in control and Cypa−/−- and CsA-treated platelets. (E) Immunoprecipitation of platelet lysates of Cypa+/+ and Cypa−/− platelets with SERCA2b Ab, followed by the detection of CyPA by Western blot analysis. To assess that a similar amount of proteins were loaded, membranes were reproved with SERCA2b Ab serving as a loading control. (F) After thrombin stimulation, platelets were lysed and immunoprecipitated with STIM1 Ab, followed by Western blot analysis with SERCA2b Ab showing colocalization of STIM1 and SERCA2b in murine platelets. STIM1/SERCA2b complex formation was also observed in CyPA-deficient platelets and on CsA treatment. (G) A5-CHO cells expressing GFP, STIM1-GFP, or STIM1S575A/S608A/S621A-GFP were allowed to adhere and spread on human fibrinogen (1 mg/mL) for 60 minutes. STIM1 was detected by the fluorescence properties of GFP. Scale bar indicates 20 μm. (H) Statistical analysis of adhesive cells per visual field. The bar graph depicts mean values ± SD (n = 10). ***P < .001.
In a recent study, Rosado et al showed that CyPA is involved in SERCA2b regulation in human platelets.18 In the present study, immunoprecipitation studies also identified colocalization of CyPA and SERCA2b in murine platelets, suggesting that CyPA is also responsible for the regulation of SERCA2b in murine platelets (Figure 4E). On thrombin stimulation, colocalization of these proteins decreased, whereas in mutant platelets, complex formation of SERCA2b and CyPA could not take place because of CyPA deficiency (Figure 4E). However, we also found colocalization of SERCA2b and STIM1 in murine platelets, providing strong evidence that SERCA2b also belongs to the protein complex STIM1/CyPA (Figure 4F). Interaction of SERCA2b and STIM1 takes place independently of CyPA, suggesting a role of STIM1 in the regulation of SERCA2b, as proposed previously by Rosado et al.18
CyPA deficiency leads to defective platelet adhesion and thrombus formation under flow conditions (Figure 1F-G). To determine whether STIM1 phosphorylation is important for cell adhesion, we made use of a platelet model cell line called A5-CHO, a specific CHO cell that stably overexpresses human integrin αIIbβ3,49 and allowed these cells to spread on fibrinogen. Decreased cell adhesion was found when STIM1 was constitutively dephosphorylated at the indicated residues (GFP 484.4 ± 21.09 and STIM1-GFP 472.8 ± 11.1 vs STIM1S575A/S608A/S621A-GFP 217.2 ± 16.3 cells per visual field; Figure 4G-H), suggesting that STIM1 phosphorylation plays a role in cell adhesion events. However, it is not clear to date whether STIM1 phosphorylation plays a role in platelet adhesion at sites of vascular injury, and must be clarified in future experiments.
CyPA-deficient mice are protected against arterial thrombosis without affecting normal hemostasis
To investigate the influence of CyPA in platelet thrombus formation in vivo, we analyzed pathologic occlusive thrombus formation by in vivo fluorescence microscopy after ferric chloride–induced mesenteric arteriole injury. After injury, the beginning of thrombus formation detected by the generation of small aggregates was comparable between Cypa+/+ and Cypa−/− mice and occurred within the first minutes after vessel injury with similar kinetics (Figure 5A). This aggregate formation resulted in complete vessel occlusion in all Cypa+/+ mice (mean occlusion time, 12.27 ± 7.1 minutes; Figure 5B-C). In contrast, stable aggregate formation and occlusive thrombus formation was abolished in Cypa−/− mice (Figure 5B-C). Although platelet aggregates were formed, they embolized to a great extent, thereby inhibiting thrombus formation and the subsequent occlusion of the injured vessel (see supplemental Videos 1 and 2). Therefore, blood flow was observed throughout the observation period of 40 minutes in all CyPA-deficient mice except for one animal (Figure 5B). This observation indicates that CyPA plays a crucial role in occlusive thrombus formation in vivo.
CyPA deficiency leads to reduced thrombus formation in vivo without affecting bleeding time. After injury of mesenteric arterioles with FeCl3, platelet adhesion and thrombus formation were monitored in vivo by fluorescence microscopy. (A-B) Time to first thrombus formation after injury (A) and time to full occlusion of the injured vessel (blood flow stopped for > 1 minute; B). Each symbol represents one animal. (C) Representative images showing the level of thrombus formation at the indicated time points. Asterisk indicates occlusion of the vessel. Scale bar indicates 50 μm. (D) Tail bleeding times for Cypa+/+ and Cypa−/− mice. Each symbol represents 1 animal.
CyPA deficiency leads to reduced thrombus formation in vivo without affecting bleeding time. After injury of mesenteric arterioles with FeCl3, platelet adhesion and thrombus formation were monitored in vivo by fluorescence microscopy. (A-B) Time to first thrombus formation after injury (A) and time to full occlusion of the injured vessel (blood flow stopped for > 1 minute; B). Each symbol represents one animal. (C) Representative images showing the level of thrombus formation at the indicated time points. Asterisk indicates occlusion of the vessel. Scale bar indicates 50 μm. (D) Tail bleeding times for Cypa+/+ and Cypa−/− mice. Each symbol represents 1 animal.
To investigate whether defective arterial thrombus formation affects normal hemostasis, tail bleeding times of Cypa+/+ and Cypa−/− mice were measured. As shown in Figure 5D, time to arrest bleeding at the site of a defined tail wound did not differ significantly between Cypa+/+ and Cypa−/− mice (8.02 ± 2.95 minutes vs 7.97 ± 2.35 minutes; P = .96), indicating that CyPA is not essential for hemostasis in the microcirculation.
Discussion
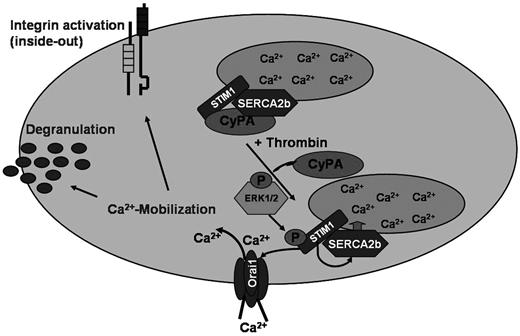
The present study unravels a novel Ca2+ regulator in platelets, the immunomodulatory-PPI CyPA. We show for the first time that CyPA deficiency leads to defective Ca2+ mobilization and Ca2+ entry responsible for multiple platelet activation defects that results in disordered thrombus formation under flow conditions in vitro and protection against arterial thrombosis in vivo. Furthermore, immunoprecipitation studies provide strong evidence that CyPA is essential for the phosphorylation of the Ca2+ sensor STIM1 (Figure 6).
CyPA is an important Ca2+ regulator in platelets. Tentative schematic illustration of CyPA-mediated regulation of Ca2+ mobilization in platelets.
CyPA is an important Ca2+ regulator in platelets. Tentative schematic illustration of CyPA-mediated regulation of Ca2+ mobilization in platelets.
Proteins of the immunophilin family group have been linked previously to Ca2+ homeostasis in different cell models. Whereas the immunophilins have been shown to regulate Ca2+ channels in the endoplasmic reticulum (eg, inositol 1,4,5-trisphosphate receptors39 ), less is known about the role of CyPs on Ca2+ reuptake.40,41 A recent study from Rosado et al described an important role for cyclophilins in the regulation of platelet SERCA2b activity and provided evidence for a direct interaction of CyPA and SERCA2b by a protein-protein interaction.18 In the presence of CsA, thrombin-induced Ca2+ mobilization was found to be modified, indicating a reduced SERCA2b activity without altering PMCA activity or other SERCA isoforms.18 Consistent with these results, the reduced Ca2+ release from intracellular stores of CyPA-deficient platelets shown in the present study suggests reduced Ca2+ levels in stores of Cypa−/− platelets and thus defective SERCA2b activity in these cells. Rosado et al found increased thrombin-induced Ca2+ mobilization from intracellular stores and we found that store release in response to all agonists tested was reduced significantly. These results were consistent with our knock-out experiments and suggest that the enzymatic activity of CyPA is responsible for the defective Ca2+ mobilization. A possible explanation for these contradictory results may be different experimental designs, but also may arise from side effects of an inhibitor such as CsA that is known to be a fungal metabolite that influences cell physiology in many ways. However, consistent with our results, Smaili et al have shown that CsA influences IP3-induced Ca2+ flux negatively and suggest that CsA did not inhibit Ca2+ flux through the IP3 receptor directly.
CyPA and STIM1 colocalize in human resting platelets, which was reduced on platelet activation.18 Our immunoprecipitation studies with murine platelets confirmed these results and suggest that this interaction is required for STIM1 phosphorylation, which was abrogated on CsA treatment and CyPA deficiency, respectively. The defective store release in CyPA-deficient platelets may result from absent STIM1 phosphorylation, which might in turn be important for SERCA2b activity and thus store refilling and store release. This hypothesis was further confirmed by immunoprecipitation studies showing an interaction of STIM1 and SERCA2b independent of CyPA. It is tempting to speculate that phosphorylation of STIM1 is important for the regulation of SERCA2b and for Orai1 and therefore for functional store release and SOCE in platelets. However, further studies are required to prove this hypothesis. In 293T and HeLa cells, binding of STIM1 to a chaperone protein, namely calnexin, was shown previously by Saitoh et al.42 The binding of STIM1/calnexin was independent of the glycosylation state of STIM1, but its relevance for STIM function in Ca2+ homeostasis remained elusive. Surprisingly, binding of STIM1 and Orai1 was not abrogated on CyPA deficiency (Figure 4C), suggesting that phosphorylation of STIM1 is not a prerequisite for the formation of a STIM1/Orai1 complex.
The identification of STIM1 as Ca2+ sensor in the endoplasmic reticulum that regulates Ca2+ entry from the extracellular compartment through its interaction with Orai1, the major SOC channel in platelets, was of great importance to understand Ca2+ homeostasis in platelets and other cells.11,12 STIM1-deficient platelets displayed a marked defect in agonist-induced Ca2+ responses, showing reduced Ca2+ release from intracellular stores and reduced Ca2+ influx in the presence of extracellular calcium11 that was similar to the Ca2+ mobilization defect observed in CyPA-deficient platelets. In contrast, platelets lacking STIM1 display platelet activation defects in response to GPVI-coupled agonists,11 whereas integrin activation and P-selectin exposure was reduced upon platelet activation with thrombin and ADP, respectively, in CyPA-deficient platelets. However, reduced thrombus formation under flow and protection against arterial thrombosis was seen in STIM1-deficient and CyPA-deficient mice, suggesting that defective Ca2+ mobilization is a prerequisite for stable thrombus formation in vitro and in vivo. In a recent study by Ahmad et al, integrin activation and P-selectin expression was reduced upon platelet activation with Cvx and PAR4-activating peptide using platelets from mice with a conditional deletion of STIM1 in platelets/megakaryocytes. In contrast to Stim1−/− and CyPA−/− platelets, thrombus formation under flow conditions was not different between platelets from Stim1fl/fl PF4-Cre and control mice. Interestingly, CyPA-deficient mice display unaltered tail bleeding times, demonstrating that CyPA is not required for normal hemostasis. In contrast, STIM1 was shown to be important for normal hemostasis, because tail bleeding times were highly variable on STIM1 deficiency.11 However, CyPA deficiency only leads to a partial defect in integrin αIIbβ3 activation that may not necessarily result in a dramatic effect on hemostasis, as shown previously in PLD1-deficient mice that were protected against arterial thrombosis but displayed normal hemostasis.47 Further studies are required to analyze the potential roles of STIM1 and CyPA in platelet activation, Ca2+ mobilization, and thrombus formation.
During store release, STIM1 aggregates and relocates to the plasma membrane, where it activates the store-operated calcium channel Orai1. Although it is known that STIM1 phosphorylation can modulate SOCE by altering STIM1 binding to store-operated calcium channels,43 the impact of this phosphorylation and conditions important to allow the phosphorylation of STIM1 were not explored. In the present study, we show that defective phosphorylation of STIM1 parallels defective SOCE in CyPA-deficient platelets. Further, we demonstrate that CyPA is the modulating protein that enables STIM1 phosphorylation on platelet activation.48 To date it is not clear how CyPA modifies engaged proteins to allow STIM1 phosphorylation. Immunoprecipitation studies indicate a direct binding of CyPA and STIM1, suggesting that CyPA induces a conformational change of the STIM1 protein to facilitate phosphorylation by the responsible kinase, but the underlying mechanisms acting directly or indirectly as prolyl isomerase or as a molecular chaperone must be addressed in further studies. However, our data clearly indicate that a possible alternative mechanism to facilitate STIM1 phosphorylation by CyPA-mediated protein modulation of the STIM1 kinase ERK1/2 was not responsible for defective STIM1 phosphorylation, because phosphorylation of ERK1/2 was comparable in CyPA-deficient and CsA-treated platelets compared with controls (Figure 4D). However, we cannot exclude that ERK1/2, although phosphorylated, is unable to transduce the signal to target proteins. In contrast, a recent study showed that secreted extracellular CyPA stimulates ERK1/2 activity in vascular smooth muscle cells.43,44 Another kinase that was shown to be regulated by the isomerase activity of CyPA is IL-2 tyrosine kinase (Itk), a protein tyrosine kinase that participates in the intracellular signaling events of Jurkat T cells.45
The results of the present study identify CyPA as a novel Ca2+ regulator in platelets important for store release and store refill and SOCE mediated by the major SOC channel Orai1. Furthermore, CyPA was shown to be important for STIM1 phosphorylation, which may account for the observed Ca2+ mobilization defects. We conclude that CyPA is an important player in Ca2+ signaling in platelets that is critical for Ca2+ mobilization and Ca2+-dependent platelet functions such as integrin activation, degranulation, thrombus formation, and stability.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Christina Flaum for excellent technical assistance.
This study was supported in part by the Tuebingen Investigative Platelet Consortium and by the Klinische Forschergruppe (KFO 274) “Platelets-Molecular Mechanisms and Translational Implications.”
Authorship
Contribution: M.E. performed the experiments, analyzed the data, designed the research, and wrote the manuscript; A.H., P.S., P.M., S.B., T.S., O.B., and F.J.M.-R., performed the experiments and analyzed the data; and F.L., A.E.M., and M.G. analyzed the data and discussed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Margitta Elvers, PhD, Medizinische Klinik und Poliklinik, Abteilung Innere Medizin III/Kardiologie und Kreislauferkrankungen, Eberhard Karls Universität Tübingen, Otfried-Müller Str 10, 72076 Tübingen, Germany; e-mail: margitta.elvers@med.uni-tuebingen.de.


![Figure 2. Intracellular CyPA regulates Ca2+ mobilization in platelets. (A-B) Defective agonist-induced Ca2+ mobilization and impaired SOCE in Cypa+/+ and Cypa−/− platelets. Fura-2–loaded Cypa+/+ (black line) and Cypa−/− (gray line) platelets before and after stimulation with thrombin (0.02 U/mL) or CRP (10 μg/mL) in the absence (0.5mM EGTA, right) or presence (1mM Ca2+, left) of extracellular Ca2+. (A) Representative tracings reflecting cytosolic Ca2+ concentration ([Ca2+]i). (B) Arithmetic mean of maximal increase in intracellular Ca2+ concentrations compared with baseline levels before stimulus (Δ [Ca2+]i ± SD, n ≥ 6 per group). *P < .05; **P < .01; ***P < .001. (C) Fura-2 fluorescence reflecting cytosolic Ca2+ concentration ([Ca2+]i) of Cypa+/+ (black line) and Cypa−/− platelets (gray line) after stimulation with 5μM thapsigargin for 10 minutes, followed by the addition of 1mM extracellular Ca2+. Representative measurements (top panel) and Δ [Ca2+]i ± SD (n ≥ 6 per group) before and after the addition of 1mM Ca2+ (bottom panel) are presented. *P < .05; ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/120/6/10.1182_blood-2011-12-398438/4/m_zh89991294990002.jpeg?Expires=1765896039&Signature=nR~rWgGlicGKIe~e2ZnrGJi0z64Xnn0J1~qonzRFYq3oT7CiDktpHSPEi6ASwWLTdz~rtb6Hp93OEttH~luROzj41IGBT5ADKsu11U8UhrCZ~RAFQN1snXJUhkUw5j4x882tGcHthxrpKx8clnX6MtnqPL-oID-Pg~AbYkNhu5VE~UNg-xuRmanS0LqzEMVz2CNwRnRZ6RRsYhqSiZXiG7MKEqlA8oNW7ePaLUsIHD9Q0FoKvHLpgXDOJxWTMCtRTjxE1dV5YNf38wXoA0UJM95qqig1wwVLSGopJSLcspwEROwnQMG36klrlEt80KfPjflx7ds68rsoqugWGIe9OQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Enzymatic activity of CyPA is responsible for defective Ca2+ mobilization. Defective agonist-induced Ca2+ mobilization and impaired SOCE in Cypa+/+ platelets pretreated with 1 μg/mL of CsA. (A) Maximal increase in cytosolic Ca2+ concentration of Cypa+/+ (black bars) and CsA-treated Cypa+/+ platelets (gray bars) after stimulation with thrombin (0.02 U/mL) and CRP (10 μg/mL), respectively. Arithmetic means of maximal [Ca2+]i ± SD (n ≥ 6 per group) before (left panel) and after (right panel) the addition of 1mM Ca2+. **P < .01; ***P < .001. (B) Representative time course of intracellular Ca2+ mobilization in Cypa+/+ (black line) and CsA-treated Cypa+/+ platelets (gray line) after exposure to 5μM thapsigargin for 10 minutes and subsequent addition of 1mM extracellular Ca2+. (C) Mean values of maximal Δ [Ca2+]i ± SD (n ≥ 5 per group) before and after the addition of 1mM Ca2+. **P < .01; ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/120/6/10.1182_blood-2011-12-398438/4/m_zh89991294990003.jpeg?Expires=1765896039&Signature=qFBXRoSlsodP-snj4F8q8dk6pL4WdaDMV0u~mZB0R-gOFC5Z2ys4n71BGdxZKMkk7xIfvJpXrAlueUn-CN4UcdNvwSDgHANtIQki5GmNVIvskBPFp2TL4ObS6nH1tweQPcmFLwUWTn8vKF8SU6OC8uL1ee7mLDWkFecnYIhKYLPIIExuLSufw9KvVSMk6K5n8fUgjs87h7xSmKrWzBYReUfihocxXPdkamZnlzR6IA4dmgDex8LuoBtDSLkYJAfbusTIglULYnwiX0Hy6Dv6wf8~qAqdaRQIy2bHQqxrIRy7RyPYWyRh24Kt7jkJhxGimXcjXfw1h9AQ4rDy9sJ~KA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal