Abstract
The calcineurin/nuclear factor of activated T cells (NFAT) signaling pathway mediates multiple adaptive T-cell functions, but recent studies have shown that calcineurin/NFAT signaling also contributes to innate immunity and regulates the homeostasis of innate cells. Myeloid cells, including granulocytes and dendritic cells, can promote inflammation, regulate adaptive immunity, and are essential mediators of early responses to pathogens. Microbial ligation of pattern-recognition receptors, such as TLR4, CD14, and dectin 1, is now known to induce the activation of calcineurin/NFAT signaling in myeloid cells, a finding that has provided new insights into the molecular pathways that regulate host protection. Inhibitors of calcineurin/NFAT binding, such as cyclosporine A and FK506, are broadly used in organ transplantation and can act as potent immunosuppressive drugs in a variety of different disorders. There is increasing evidence that these agents influence innate responses as well as inhibiting adaptive T-cell functions. This review focuses on the role of calcineurin/NFAT signaling in myeloid cells, which may contribute to the various unexplained effects of immunosuppressive drugs already being used in the clinic.
Introduction
Ligation of pattern recognition receptors (PRRs) results in the activation of the calcineurin/nuclear factor of activated T cells (NFAT) pathway in myeloid cells, as was first revealed by our discovery that dendritic cells (DCs) produce IL-2 after encountering microbial products.1,2 NFAT-regulated genes have subsequently been shown to modulate the functions of a broad range of myeloid cells, including macrophages, mast cells, megakaryocytes, and osteoclasts. In mice, NFAT signaling has now been shown to drive neutrophil-mediated resistance to Candida albicans,3 and IL-2 production by DCs challenged with bacteria has been demonstrated both in vitro and in vivo, resulting in the activation of NK cells4,5 and regulatory T cells (Tregs).6,7 NFAT-dependent genes also modulate DC life cycle in response to lipopolysaccharide (LPS) activation, leading to the apoptosis of terminally differentiated DCs.8 In addition, NFAT signaling has been shown to negatively regulate myeloid cell development.9 Accordingly, NFAT expression levels are down regulated during myeloid differentiation of hematopoietic CD34+ stem cells (HSCs).10 These data indicate that, in addition to better-known roles for NFAT in lymphoid development,11 the calcineurin/NFAT pathway plays a critical role in the development and function of innate myeloid cells.
Calcineurin/NFAT inhibitors, such as cyclosporine A (CsA) and tacrolimus (FK506), are primarily used to prevent allograft rejection and to treat GVHD after bone marrow transplantation. However, these drugs are also used to treat a wide variety of immune disorders that include atopic dermatitis and refractory colitis. Both CsA and FK506 are still broadly used to block the calcineurin/NFAT pathway, although some effects in the modulation of other pathways have been reported. The newly discovered roles played by NFAT in the function of innate immune cells may contribute to the higher rates of infection observed in patients who receive calcineurin inhibitors or other immunosuppressive drugs. This review summarizes our current knowledge of NFAT functions in innate myeloid cells, with a particular focus on NFAT signaling in immune homeostasis and in the early phase of the innate response.
Calcineurin/NFAT signaling
NFAT is composed of a family of transcription factors that includes 5 members, 4 of which are regulated by Ca2+ signaling; NFAT1 (NFATc2 or NFATp), NFAT2 (NFATc or NFATc1), NFAT3 (NFATc4), NFAT4 (NFATx or NFATc3), and calcineurin-independent NFAT5 (TonE-BP or NFATL1). Calcineurin is a key phosphatase that facilitates the translocation of NFAT molecules from the cytoplasm into the nucleus to regulate diverse cellular functions, including proliferation, differentiation, and development. NFAT was first identified more than 20 years ago as an inducible transcription factor that could bind to the IL-2 promoter to activate cytokine production after T-cell activation.12 The NFAT pathway has since been studied extensively in lymphocytes, but a key role for this signaling pathway in myeloid cells has now also been identified.3,8,9
The classic calcineurin/NFAT signaling pathway in lymphocytes has been reviewed in detail elsewhere.11,13 In brief: on antigen engagement of lymphocyte receptors, phospholipase C-γ (PLC-γ) becomes activated and hydrolyzes phosphatidylinositol-4,5-bisphosphate into inositol-1,4,5-trisphosphate (IP3) and diacylglycerol. IP3 then binds to specific receptors on the endoplasmic reticulum and drives Ca2+ release from the endoplasmic reticulum into the cytoplasm, which triggers STIM1 and Orai1-mediated opening of Ca2+ release-activated Ca2+ channels. As a result of increased intracellular Ca2+, the calcineurin enzyme becomes active and dephosphorylates NFAT, allowing NFAT translocation into the nucleus and subsequent regulation of gene expression. It should be noted, however, that NFAT must ultimately bind to additional transcription factors, such as AP1 to regulate gene expression. In DCs, LPS engagement of Toll-like receptor 4 (TLR4) and CD14 activates Src-family kinase and PLC-γ2 to drive the production of IP3 and diacylglycerol8,14 (Figure 1). Interestingly, smooth LPS-mediated activation of DCs is fully dependent on an extracellular source of Ca2+8,14 ; and hence, the authors speculate that DCs, unlike lymphocytes, may not use Ca2+ derived from the endoplasmic reticulum to support activation.14,15 Instead, IP3 may bind to cognate receptors on the plasma membrane to open Ca2+ channels, leading to Ca2+ influx and activation of calcineurin/NFAT signaling (Figure 1). A similar mechanism for cell activation has also been suggested in B cells.16
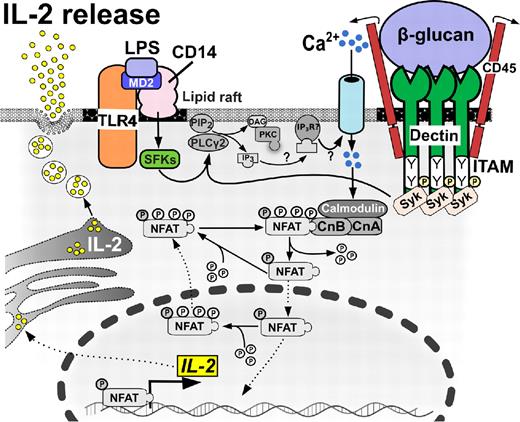
Calcineurin/NFAT-dependent IL-2 release in DCs. DCs produce IL-2 early after encountering bacterial or fungal cell wall components. Ligand binding to dectin 1 signals through ITAM-like motifs, leading to the recruitment of Syk family kinases and Ca2+ influx, which culminates in NFAT translocation to the nucleus, gene transcription, and extracellular release of IL-2 cytokine. Alternatively, ligand binding to CD14 requires collaboration of MD2 and Src-family kinases (SFKs) with TLR4 molecule to initiate this pathway.
Calcineurin/NFAT-dependent IL-2 release in DCs. DCs produce IL-2 early after encountering bacterial or fungal cell wall components. Ligand binding to dectin 1 signals through ITAM-like motifs, leading to the recruitment of Syk family kinases and Ca2+ influx, which culminates in NFAT translocation to the nucleus, gene transcription, and extracellular release of IL-2 cytokine. Alternatively, ligand binding to CD14 requires collaboration of MD2 and Src-family kinases (SFKs) with TLR4 molecule to initiate this pathway.
A key function of NFAT in immune cells is to regulate the expression of potent immunomodulatory cytokines. In T cells, the downstream targets of NFAT include IL-2 growth factor, which regulates lymphocyte development and activation and may contribute to the overall function of Tregs by regulating their expansion, differentiation, and survival.7,17 NFAT1 also regulates the transcription of the polarizing cytokines IFN-γ and IL-4, which drive Th1/Th2 cell differentiation.18,19 The distal cis-regulatory element of IL-10 cytokine is also bound by NFAT1 and interferon regulatory factor-4 in activated Th1 and Th2 cells.20 Similarly, IL-10 expression can be activated through a Ca2+ signaling pathway in B cells on B-cell receptor stimulation.21 Production of the Th1 cytokine subunit IL-12p40 has been shown to be induced by the cooperation of NFAT with ICSBP in RAW-264.7 cells activated with LPS and IFN-γ.22 NFAT has also been reported to regulate several key modulators of innate immune responses, including the production of IL-3, which is required for T cell and myeloid lineage differentiation.23 Furthermore, TNF-α in T cells is directly regulated by NFAT1 and NFAT2 through promoter occupation.24 Indeed, GM-CSF plays an essential role in myeloid cell development and is a direct transcriptional target of NFAT.25 Other downstream targets of NFAT in innate immune cells26 include early growth response 2 and 3 (Egr2, Egr3), and cyclo-oxygenase-2 (Cox2), which are involved in angiogenesis and inflammation.
NFAT phosphorylation state and export from the nucleus are critically regulated by several enzymes including casein kinase, glycogen synthase kinase 3, P38, and JUN N terminal kinase, as has been reviewed elsewhere.17 A noncoding RNA NRON scaffold complex was recently reported to suppress NFAT translocation into the nucleus.27,28 The NRON scaffold is composed of a long intergenic noncoding RNA, a scaffold protein, an IQ motif containing GTPase activating protein, and 3 NFAT kinases (casein kinase, glycogen synthase kinase 3, and dual specificity tyrosine phosphorylation-regulated kinase). Loss of function experiment suggests that both long intergenic noncoding RNA and IQ motif containing GTPase activating protein are essential for NFAT phosphorylation.27,28 Intriguingly, leucine-rich repeat kinase 2 (LRRK2) was recently identified by genome-wide association studies as a major susceptibility gene for Crohn disease, and LRRK2 reportedly forms part of the NRON complex to negatively regulate NFAT activity in bone marrow–derived macrophages.29
Microbial-dependent activation of calcineurin/NFAT signaling in myeloid cells
Numerous cells of the innate immune system contribute to early antimicrobial immune responses and can subsequently influence adaptive immunity. Innate leukocytes use a range of receptors that recognize microbial patterns and trigger transcriptional changes in response to microenvironmental perturbations during infections. Among several distinct subsets of myeloid antigen-presenting cells, DCs play a particularly important role in instructing the adaptive immune system after microbial recognition through PRRs. After microbial encounter, DCs migrate to the lymph nodes to instruct adaptive T-cell responses before undergoing terminal differentiation and ultimately dying by apoptosis.30 Subsets of tissue-resident myeloid antigen-presenting cells then further shape local T-cell differentiation to promote homeostasis in the peripheral tissues.31
Numerous studies have demonstrated that LPS binding to CD14 is enhanced by LPS binding protein, which is followed by CD14-mediated transfer of LPS to the TLR4/MD2 complex and subsequent activation of the intracellular cascade through adaptors MyD88-TIRAP and TRAM-TRIF. The association of these 2 different pathways leads to the early activation of NFκB and the late activation of interferon regulatory factor-3 responses.32 CD14 activation after LPS engagement is mediated by lipid rafts and leads to Src kinase activation.33 The resultant Ca2+ mobilization leads to the dephosphorylation of NFAT by calcineurin and promotes translocation of the active form of NFAT into the nucleus. Exposure to LPS is thus able to increase the translocation of NFAT in DCs, leading to increased production of IL-2. Whereas smooth LPS-induced Ca2+ mobilization and subsequent IL-2 production are dependent on CD14, TLR4 is not required to mediate DC activation.8 The truncated form or rough LPS is able to induce Ca2+ flux via binding to TLR4 alone, as clearly demonstrated in CD14-deficient DCs.14 CD14 transfers LPS to the TLR4-MD-2 complex and promotes TLR4 oligomerization. Recently, CD14 has also been reported to contribute to LPS endocytosis on TLR4 binding. This phenomenon is mediated by Syk and PLC-γ2, facilitates the intracellular activation of TRIF, and culminates in the production of type I IFN (Figure 2).34 Other PLC-γ2–dependent pathways are also likely to become activated by this signaling cascade.
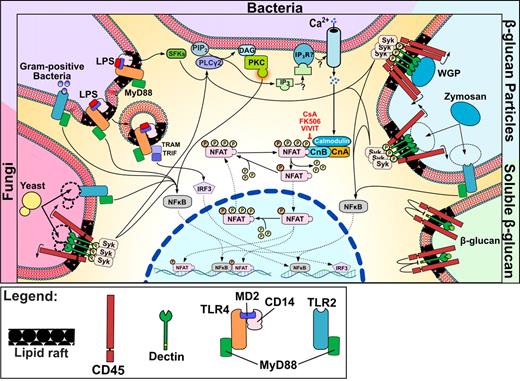
NFAT signaling in DCs in response to microbes, TLR ligands, and particulates. Schematic representation of a DC activated by different stimuli. Microbial stimuli, both bacterial and fungal, activate Ca2+ flux, which promotes NFAT dephosphorylation through close collaboration with TLR signaling. Stimulation also occurs in response to particulate β-glucan, being recognized by the dectin 1 clusters that are required for Syk pathway activation and NFAT nuclear translocation.
NFAT signaling in DCs in response to microbes, TLR ligands, and particulates. Schematic representation of a DC activated by different stimuli. Microbial stimuli, both bacterial and fungal, activate Ca2+ flux, which promotes NFAT dephosphorylation through close collaboration with TLR signaling. Stimulation also occurs in response to particulate β-glucan, being recognized by the dectin 1 clusters that are required for Syk pathway activation and NFAT nuclear translocation.
The calcineurin signaling capacity of DCs has now been tested in response to a diverse array of pathogen-associated molecular pattern ligands. In particular, bacterial CpG oligonucleotides and Pam3CyS (which activate TLR9 and TLR2, respectively) have each been shown to increase IL-2 production by DCs. In contrast, proinflammatory stimuli, such as PGE2, are not able to trigger IL-2 production by DCs.35 Both Leishmania mexicana promastigotes and whole bacillus Calmette-Guerin can trigger IL-2 synthesis by DCs after only 24 hours of coculture,35 and marked IL-2 production by DCs has also been observed on infection with Escherichia coli.4 Fungal cell wall components rich in repeated β-D-glucose units (usually connected by β-1,3-glycosidic linkages and referred to as “β-D-glucan”) also activate the intracellular Ca2+/calcineurin pathway in DCs.26,36 It was previously reported that after macrophage ingestion of fungus, released β-glucan binds to complement receptor 3 on neutrophils and facilitates the killing of tumor cells opsonized by C3b.37 Consequently, β-glucan is now regarded as a “biologic response modifier” because of the ability of this molecule to increase antitumor neutrophil responses,37 facilitate phagocytosis,38 prevent infections,39 and provide therapeutic benefit in arthritis. However, β-glucan treatment of arthritis symptoms is somewhat controversial because high-dose administration of zymosan or curdlan can exacerbate arthritis in genetically predisposed mice.40 This discrepancy can perhaps be attributed to the fact that different β-glucan molecules exert different biologic effects according to their size, structure, and water solubility and different receptor recognition.41 For example, zymosan is well known to bind to TLR242 and is also recognized by the dectin 1 receptor on myeloid cells, leading to Ca2+ mobilization and NFAT translocation.26,43
Dectin 1 is a pattern recognition receptor expressed primarily by myeloid cells and constitutes a member of the C-type lectin receptor (CLR) family. The CLR family includes dectin 1 (also known as CLEC7A), dectin 2 (CLEC6A), mincle (CLEC4E), DC-specific ICAM-3–grabbing nonintegrin (DC-SIGN), mannose receptor, langerin (CLEC4K) and mannose-binding lectin.44 On β-glucan binding to dectin 1, Src kinases phosphorylate the dectin 1 ITAM-like motifs to create a docking site for Syk and thus drive PLC-γ2 activation, which then either stimulates NFAT26,45 or activates the CARD9-BCL10-MALT1 complex. This complex regulates nuclear translocation of the NF-κB subunit p65 and the activation of the canonical NF-κB pathway. It has also been shown that dectin 1 and TLR2 can cross-regulate in a Syk-independent manner, suggesting that dectin 1 may also modulate TLR signaling. There is also evidence that dectin 1 signaling leads to the activation of the noncanonical NF-κB pathway through Raf-1, which seems to be involved in cross-talk between TLR2 and dectin 1.46 Interestingly, both TLR2/MyD88 and dectin 1/Syk signaling synergize for optimal production of IL-2 by DCs.47 Moreover, zymosan and C albicans yeast are able to trigger dectin 1/NFAT-dependent expression of Egr2 and Egr3 in macrophages and DCs independently of TLR2. Dectin 1 signaling also activates NFAT-regulated genes in DCs and macrophages in response to zymosan, but not in response to laminarin, emphasizing the poor ability of soluble forms of β-glucan to trigger Ca2+ mobilization and induce NFAT activation. Removal of TLR-activating motifs from zymosan does not prevent NFAT activation by this molecule, suggesting that TLR stimulation and NFAT stimulation are independent events, even though collaboration between these pathways does seem to occur. Conversely, production of IL-6 and TNF-α is mediated by dectin 1 and TLR2 only and does not require NFAT signaling. It has also been demonstrated that PLC-γ2 activates Ca2+ flux in DCs exposed to zymosan, leading to activation of the CARD9/BCL10/MALT9 complex and subsequent NF-κB activation. PLC-γ2 thus appears to act on alternative pathways in addition to influencing calcineurin/NFAT signaling, which suggests a complex role for PLC-γ2 in the activation of myeloid lineage cells.48 These data suggest a possible regulatory role for NFAT in myeloid cells exposed to fungi. Of note, the germinated hyphal form of Candida is unable to increase Egr expression in DCs because of low surface expression of β-glucan and corresponding poor ability to activate dectin 1.26 β-glucan activation of NFAT signaling in DCs and macrophages was recently confirmed using different molecular weight fractions of soluble and insoluble Saccharomyces cerevisiae β-1,3/1,6-glucans. These data showed that only particulate β-glucan, which lacked TLR-stimulating capacity, was able to trigger the NFAT pathway through Syk phosphorylation. In contrast, β-glucan polymers were unable to activate dectin 1 signaling.49
After DC activation with β-glucan derivatives such as zymosan, dectin 1 localizes in the lipid rafts of the plasma membrane in an analogous manner to CD14. Syk and PLC-γ2 are also recruited to lipid rafts on activation of dectin 1, and disruption of lipid rafts leads to reduced Ca2+ flux, decreased IL-2 production, and incomplete phagocytosis of zymosan by DCs.50 The internalization of particulate β-1,3/1,6-glucans triggers signals that are therefore reminiscent of “frustrated phagocytosis,” which requires Ca2+ mobilization in the early phases of the internalization process.51 Activation of the calcineurin pathway during this specific phase of DC activation may exert different effects from the phagocytosis process itself, as well as influencing inflammatory responses derived from the “frustrating” internalization of particulates (Figure 2).
Functional role of NFAT in innate immune cells
Numerous DC functions are activated by PRRs that induce the transcription of genes regulated by NF-κB and AP1. These transcription factors are responsible for regulating DC maturation, cytokine production, and cell survival. Moreover, recent reports have shown that NFAT signaling also plays a key role in innate cell activation. DCs are capable of priming NK cells,52,53 and the activation of NK cells can be further enhanced by DC production of IL-2.4,5 DCs are the main cell type that bridges innate and adaptive immunity, and DC-derived IL-2 can help to maintain Tregs, as has been reported in spontaneous type I diabetes in nonobese diabetic mice.54 NFAT signaling also controls the expression of genes that mediate DC apoptosis in response to LPS, which is a key regulatory mechanism that limits excessive immune activation.8
Previous reports have indicated that failure of DC apoptosis may promote the development of autoimmunity.55,56 In vivo, DC subsets differ in their longevity, but most are usually cleared by apoptosis within 3 days,57 and this rapid turnover of DCs is further increased on TLR stimulation. Zanoni et al identified several genes (Nur77, Gadd45g, Ddit3, and CHOP-10) that are responsible for apoptosis induction after DC stimulation with LPS,8 and a proapoptotic role for NFAT1 has also been reported in T and B cells.58,59
Macrophages are a population of professional phagocytes that express NFAT1, NFAT2, NFAT4,3 and NFAT560 (Table 1). Calcineurin has been shown to act as a negative regulator of NF-κB signaling in steady-state macrophages,61,62 whereas macrophages either treated with tacrolimus or impaired in calcineurin signaling have higher activation of NF-κB and therefore produce higher levels of IL-12 and TNF-α.61,62 Furthermore, impairment of calcineurin signaling in macrophages leads to a state of insensitivity to LPS, which suggests a role for calcineurin in mediating LPS tolerance.63 Mice administered with lethal doses of LPS exhibit enhanced survival if treated with tacrolimus, and murine macrophages deficient in calcineurin subunits exhibit an LPS-tolerant phenotype, which has led to the identification of calcineurin-driven LPS tolerance as a potential mediator of protection against LPS toxicity.63 Although the authors did not identify a direct role for NFAT in this process, these findings indicate that inhibition of NFAT may have multiple consequences across the innate immune compartment. Accordingly with those findings, the VIVIT peptide64 also inhibits IL-12 and TNF-α expression in LPS-stimulated macrophages,65 and the authors have also demonstrated a beneficial effect of calcineurin/NFAT inhibition for amelioration of experimental colitis. A similar observation has been done by Liu et al,29 who showed that zymosan-stimulated macrophages express high levels of IL-6 and IL-12 in a NFAT-dependent manner using LRRK-2–deficient mice. This is an important finding because LRRK2 is a highly specific regulator of NFAT activity. LRRK-2 deficiency causing NFAT hyperactivation provides proof of the positive stimulatory effects of NFAT on cytokine production in macrophages. Furthermore, the finding that CD14 signaling triggers the calcineurin/NFAT pathway in DCs but not in macrophages,8 strongly suggests that NFAT signaling plays distinct roles in different cell types. Whereas DCs undergo apoptosis early after activation, stimulated tissue macrophages can continue to sustain inflammation for extended periods of time. Despite DCs and macrophages sharing similar patterns of innate receptors, signal transduction in each of these cell types may lead to very different outcomes (Figure 3; Table 1).
Different functions of NFAT transcription factors in innate cells
| Cell type . | NFAT . | Function . | Reference(s) . |
|---|---|---|---|
| DCs | NFAT1 NFAT2 | Regulation of the DC life cycle/apoptosis of terminally differentiated DCs in response to LPS | 8 |
| Regulation of IL-2, IL-10, and IL-12 expression | 1,4,26,35 | ||
| Activation of NK cells | 4,5 | ||
| Macrophages | NFAT1, | Regulation of IL-6, IL-10, and IL-12 and TNF-α expression | 5,26,29,65 |
| NFAT2, NFAT4, NFAT5 | Regulation of multiple TLR-induced genes (eg, Nos2, inducible nitric oxide synthase [iNOS]) | 60 | |
| Mast cells | NFAT1, | Regulation of mast cell activation during hypoxia | 74 |
| NFAT2 | Enhanced mast cell survival after FcϵRI activation resulting from regulation of A1 expression | 76,77 | |
| Regulation of IL-13 and TNF expression | 110 | ||
| Neutrophils | NFAT2, NFAT4 | Regulation of IgE-mediated expression of cyclo-oxygenase and release of prostaglandin | 82 |
| Resistance to C albicans infection; genes involved include IL-10, Cox2, Egr1, and Egr2 | 3 | ||
| Eosinophils | NFAT1, | Degranulation, cytokine release, apoptosis | 84,88 |
| NFAT2 | |||
| Basophils | NFAT2 | Regulation of IL-4 production | 83,91 |
| Megakaryocytes | NFAT2, | Role in megakaryocyte differentiation | 10 |
| NFAT3 | Mediates CD154 expression | 111 | |
| Osteoclasts | NFAT2 | Master regulator of osteoclastogenesis | 69,112 |
| NK cells | NFAT1, | Inducible NFAT expression | 92 |
| NFAT2 | Mediates CD16-induced activation of cytokine genes | 94,95 | |
| Role in cytotoxicity and cell proliferation | 94,96 |
| Cell type . | NFAT . | Function . | Reference(s) . |
|---|---|---|---|
| DCs | NFAT1 NFAT2 | Regulation of the DC life cycle/apoptosis of terminally differentiated DCs in response to LPS | 8 |
| Regulation of IL-2, IL-10, and IL-12 expression | 1,4,26,35 | ||
| Activation of NK cells | 4,5 | ||
| Macrophages | NFAT1, | Regulation of IL-6, IL-10, and IL-12 and TNF-α expression | 5,26,29,65 |
| NFAT2, NFAT4, NFAT5 | Regulation of multiple TLR-induced genes (eg, Nos2, inducible nitric oxide synthase [iNOS]) | 60 | |
| Mast cells | NFAT1, | Regulation of mast cell activation during hypoxia | 74 |
| NFAT2 | Enhanced mast cell survival after FcϵRI activation resulting from regulation of A1 expression | 76,77 | |
| Regulation of IL-13 and TNF expression | 110 | ||
| Neutrophils | NFAT2, NFAT4 | Regulation of IgE-mediated expression of cyclo-oxygenase and release of prostaglandin | 82 |
| Resistance to C albicans infection; genes involved include IL-10, Cox2, Egr1, and Egr2 | 3 | ||
| Eosinophils | NFAT1, | Degranulation, cytokine release, apoptosis | 84,88 |
| NFAT2 | |||
| Basophils | NFAT2 | Regulation of IL-4 production | 83,91 |
| Megakaryocytes | NFAT2, | Role in megakaryocyte differentiation | 10 |
| NFAT3 | Mediates CD154 expression | 111 | |
| Osteoclasts | NFAT2 | Master regulator of osteoclastogenesis | 69,112 |
| NK cells | NFAT1, | Inducible NFAT expression | 92 |
| NFAT2 | Mediates CD16-induced activation of cytokine genes | 94,95 | |
| Role in cytotoxicity and cell proliferation | 94,96 |
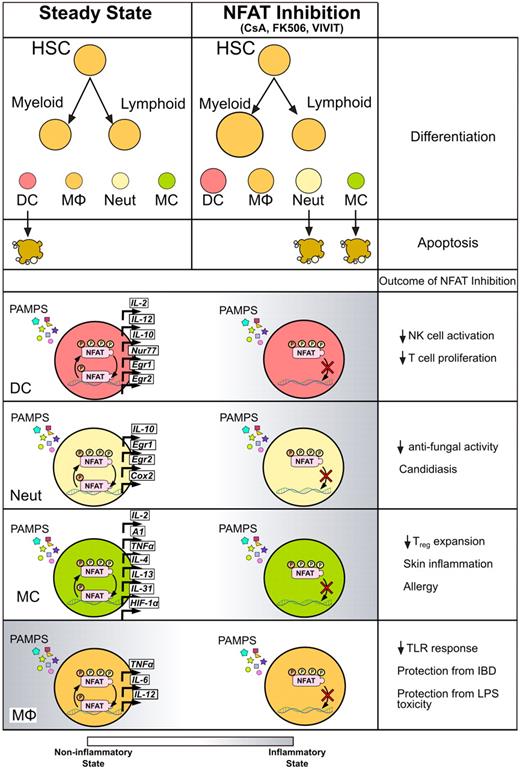
Effects of calcineurin/NFAT inhibitors on myeloid innate immune cells. Treatment with CsA, tacrolimus, or VIVIT affects the myeloid cells at different levels. Inhibition of NFAT enhances the development of myeloid cells from hematopoietic precursors as well as several immune functions in differentiated cell types. After exposure to different PAMPs, innate myeloid cells respond by activating NFAT and transcribing downstream genes, resulting in modulation of proinflammatory and prosurvival gene programs. Treatment with CsA or tacrolimus during the response to microbial stimuli decreases NFAT nuclear translocation, leading to changes in inflammatory status of different types of myeloid cells, eventually exacerbating or protecting from infections or inflammatory syndromes.
Effects of calcineurin/NFAT inhibitors on myeloid innate immune cells. Treatment with CsA, tacrolimus, or VIVIT affects the myeloid cells at different levels. Inhibition of NFAT enhances the development of myeloid cells from hematopoietic precursors as well as several immune functions in differentiated cell types. After exposure to different PAMPs, innate myeloid cells respond by activating NFAT and transcribing downstream genes, resulting in modulation of proinflammatory and prosurvival gene programs. Treatment with CsA or tacrolimus during the response to microbial stimuli decreases NFAT nuclear translocation, leading to changes in inflammatory status of different types of myeloid cells, eventually exacerbating or protecting from infections or inflammatory syndromes.
Oscillation of intracellular Ca2+ levels has been described as an important regulatory mechanism in various different cell types and can modulate the differentiation of mesenchymal stem cells,66 as well as influencing macrophage and DC development. The oscillation of Ca2+ levels during “frustrated phagocytosis” was first described almost 15 years ago,51 but the molecular mechanisms underpinning this phenomenon were uncovered only recently. Vukcevic et al have observed strong steady-state oscillation of intracellular Ca2+ flux in immature DCs followed by NFAT2 translocation.67 This oscillation was lost on DC maturation, leading the authors to speculate that Ca2+ flux contributes to the maintenance of an immature DC phenotype. It was also recently shown that Ca2+ flux in macrophages could be induced by prolonged exposure to TNF-α, which can occur during chronic inflammation in diseases, such as arthritis. ITAM-containing receptors appear to be the main inducers of downstream calcineurin/NFAT signaling in this instance.68,69
Mast cells are another subset of innate immune cells that is capable of producing IL-2. Mast cells drive allergic inflammation and exert a potent influence on adaptive immune responses.70,71 Cytokine production by mast cells appears to be influenced by NFAT, and coculture of mast cells with DCs leads to increased expression of cytokines, including NFAT-dependent IL-2.72 In a murine model of chronic allergic dermatitis, mast cell–derived IL-2 successfully ameliorated disease by enriching for Tregs and suppressing skin inflammation.73 Mast cell activation during hypoxia has also been shown to depend on NFAT signaling.74 Activation of high affinity IgE receptor (FcϵRI) leads to the release of regulatory factors stored in mast cell granules,75 which can enhance mast cell survival.76,77 Recently, the mechanism of this prolonged survival has been linked to the NFAT-dependent transcription of the antiapoptotic Bcl2 family member gene A1/Bfl-1.78 The antiapoptotic function of A1 has also been described in neutrophils and macrophages.79-81 IgE crosslinking on mast cells leads to Ca2+ flux followed by NFAT translocation and up-regulation of A1 expression, which may explain previous data indicating that increased Ca2+ levels are beneficial to mast cell survival.77
NFAT signaling has now been demonstrated in several different types of granulocytes (Table 1). NFAT expression was first described as an important inducer of inflammatory responses in human neutrophils,82 which express both NFAT2 and NFAT4. IgE-mediated neutrophil expression of cyclo-oxygenase and release of prostaglandins are regulated by NFAT translocation.82 Expression of IL-10, Cox2, Egr1, and Egr2 genes is impaired in mice that conditionally lack calcineurin subunit B in neutrophils. Each of these genes is dependent on calcineurin/NFAT signaling and exerts an important role in innate immune responses.3 Surprisingly, knockdown of NFAT1 and NFAT2 expression in DCs does not affect the ability of these cells to maintain IL-10 expression.3 Neutrophils deficient in calcineurin signaling are unable to protect mice from C albicans infection,3 and NFAT-impaired neutrophils lose the ability to kill Candida in vitro, whereas classic antifungal activities, such as myeloperoxidase release and nitric oxide production, remain functional (Figure 3).
The active NFAT pathway in neutrophils is comparably active in basophils83 and eosinophils.84 Eosinophils constitutively express NFAT1 and NFAT285 and can synthesize many NFAT-dependent molecules, including IL-286 and GM-CSF87 on activation. Treatment with calcineurin/NFAT inhibitors leads to impaired degranulation and cytokine release as well as prolonged eosinophil survival.88 Despite constituting only a minor subset of myeloid cells, basophils play important roles in allergic reactions that are mainly exerted through the production of IL-4.89,90 Calcineurin/NFAT signaling regulates basophil IL-4 synthesis,91 and a recent study showed that crosslinking of FcϵRI receptors on basophils promotes Ca2+ signaling and IL-4 transcription. Together, these data indicate that the majority of myeloid cell types are able to signal through the calcineurin/NFAT pathway and that the outcome of signaling is ligand-specific and cell type-dependent (Figure 3).
Although from lymphoid lineage, NK cells play important roles in innate immune responses. NK cells express both NFAT1 and NFAT2 on CD16 ligand stimulation,92 leading to the transcription of NFAT-dependent cytokines, including TNF, GM-CSF, and IFN-γ.93 This finding may have important implications for calcineurin inhibitor therapies because NK cell–mediated cytokine production is a critical component of effective immune surveillance. The calcineurin/NFAT pathway becomes activated when NK cells are stimulated with IL-2.94 NFAT signaling in IL-2–treated NK cells contributed to the enhanced expression of Ca2+-dependent cytokines GM-CSF, TNF, TGF-β, and also cyclin-dependent kinase inhibitor 1 (p21). Wang et al have reported that a high proportion of CD56+ NK cells lack the expression of CD16 and killer-cell immunoglobulin-like receptor after culture in the presence of CsA.95 Both Ca2+ flux and NFAT translocation were observed in these NK cells during their interaction with target cells,95 and CsA exposure markedly increased NK cell cytotoxic potency. In contrast, Kim et al have reported the inhibition of proliferation and cytotoxic function in NK cells isolated from bone marrow transplant patients undergoing tacrolimus treatment.96 Kim et al also identified a decrease in NK cell expression of cytokines and adhesion molecules,96 which is consistent with the findings of Wang et al NK cells that infiltrate grafted tissues may thus be modulated by concurrent therapies in the host,95 which could have profound implications for the course of GVHD as well as solid organ transplantation.
NFAT signaling confers innate immune protection and regulates homeostasis
The calcineurin inhibitors are generally administered in patients with immune-mediated disorders with the aim of inducing a state of general immunosuppression. The immunosuppressive state induced by these drugs is mainly achieved by inhibition of T-cell responses and has proven invaluable in reducing the risk of rejection after solid organ transplantation. Although widely accepted that the immunosuppression induced by calcineurin inhibitors is the result of the blockade of NFAT translocation, which inhibits T-cell cytokine production and limits the release of IL-2, the effects of CsA have now also been studied in detail in myeloid cells. These studies aimed to explain the increased rates of infection observed after administration of NFAT inhibitors in organ-transplanted patients. In particular, treated allograft recipients frequently undergo fungal and viral infections, which increase the risk of transplant rejection.97 Intriguingly, treatment with CsA can increase susceptibility to C albicans infection in mice that lack conventional lymphocytes,3 suggesting that the drug must also act on nonlymphoid cell types. These studies further showed that NFAT inhibition results in reduced production of anti-inflammatory cytokines, including IL-10, which is critical for the resolution of inflammation after fungal infection.98 Taken together with the observation that splenic DCs deficient in NFAT1 and NFAT2 are defective in IL-10 production in response to zymosan, these data suggest that NFAT can support anti-inflammatory responses in myeloid cells during fungal infection.98 Recently, NFAT signaling in myeloid cells was found to regulate inflammation through the kinase LRRK2.29 This enzyme modulates the interaction between NFAT and the NRON complex, which blocks NFAT translocation to the nucleus independently of phosphorylation events. LRRK2-mediated NFAT retention in the cytoplasm occurs in response to zymozan challenge in bone marrow–derived macrophages. Zymozan stimulation of macrophages regulates the level of LRRK2 in the cytoplasm, thus indirectly regulating NFAT translocation. LRRK2 deficiency leads to pronounced NFAT translocation in response to microbial stimuli, including LPS and zymosan. In the absence of LRRK2, macrophages produce very high levels of inflammatory cytokines, including IL-12 and IL-6, which can be reduced by exposure to NFAT inhibitors, such as CsA.
Taken as a whole, these data indicate that myeloid cell inflammatory responses can be profoundly modulated by drugs that inhibit NFAT signaling and thus have significant implications for the clinic. Interestingly, CsA inhibited the severity of colitis in LRRK2-deficient mice, which underlines an important role for LRRK2 in regulating inflammation through NFAT. This recent observation suggests that genetic deficiency in LRRK2 may decrease the amount of available NFAT protein, which leads to dysregulated NFAT activation in innate immune cells, and potentially contributes to the increased risk of Crohn disease in genetically predisposed persons.29 Moreover, these data suggest that the critical role of NFAT in immune regulation is achieved during the early phases of innate immune activation. Indeed, NFAT-dependent IL-2 production in DCs occurs within 4 to 8 hours of PRR activation and can promote IFN-γ expression by NK cells. Notably, IL-2 has also been reported to regulate Th2 differentiation in a STAT5-dependent manner,99 and may therefore also contribute to allergic inflammatory responses. Furthermore, NFAT-expressing DCs may promote Treg activation6 because DC-derived IL-2 is required for Treg expansion in both in vitro and in vivo systems.7,100
Findings that several NFAT family members are expressed by HSCs during differentiation toward some myeloid lineages have revealed a novel role for NFAT in the regulation of innate immune homeostasis. The role of NFAT1 in hematopoiesis has been demonstrated only recently.101 Kiani et al have shown that NFAT is expressed during HSC differentiation toward megakaryocytes, whereas differentiation toward granulocytes and erythroid cells does not require NFAT expression.10,102 Lack of NFAT leads to erythropenia, reduced numbers of hematopoietic progenitors, and later to reduced bone marrow hematopoiesis. Interestingly, NFAT has now also been detected in stromal stem cells as well as in HSCs.10,103
NFAT regulation of immune homeostasis is mediated by growth factors, such as GM-CSF and IL-2, which are transcribed after NFAT translocation.25 Homeostasis of innate immune cells is a tightly regulated process that depends on growth factors, including GM-CSF104 and Flt3-L.105 Systemic administration of calcineurin/NFAT inhibitors may therefore profoundly impact innate immune homeostasis in treated patients. Indeed, GM-CSF, IL-2, and IFN-γ levels in serum decrease only 2 hours after inhibitor administration in immunosuppressed patients.106 These effects may have an immediate impact on innate responsiveness because GM-CSF drives the development of DCs and granulocytes as well as regulating the release of neutrophils from the bone marrow.104 We recently identified NFAT as a negative regulator of myeloid development using the VIVIT peptide to inhibit NFAT signaling in HSCs that were then used to reconstitute an irradiated host.9 Compared with their control counterparts, the myeloid cells that developed from NFAT-impaired progenitors displayed an advantage in repopulating the myeloid compartment (Figure 3). These data also indicate that NFAT signaling participates in the negative regulation of the myeloid compartment by modulating genes that influence cell cycle progression and apoptosis.9 Accordingly, proliferation and differentiation of skin stem cells are enhanced by exposure to CsA because their cell cycle is negatively regulated by NFAT.107
Further studies have to address the roles of NFAT considering its complexity in expression and function. Thus, the newly described role of NFAT during myeloid cell development, together with the overall positive or negative effects of NFAT signaling among differentiated cells, has to be considered. While affecting hematopoiesis as well as both innate and adaptive immune responses, calcineurin/NFAT inhibitors might contribute to the outcome of treatment including higher susceptibility to infections.
Conclusion
Recent findings have confirmed that the role of NFAT signaling in the regulation of immune responses is broader than that originally described only in conventional T cells. NFAT is now known to contribute to hematopoiesis as well as innate and adaptive immune functions in a variety of myeloid lineage cells. Increasing evidence also points to a key role for the calcineurin/NFAT pathway in innate immune homeostasis.
These findings have improved our understanding of the molecular mechanisms that underpin various side effects of NFAT inhibitors that are used in the clinic, including perturbation of immune homeostasis and increased susceptibility to infections and malignancies. Recent data from our laboratory have clearly indicated that NFAT negatively regulates myeloid differentiation and that inhibition of calcineurin/NFAT signaling enhances the development of myeloid cells.9 It is therefore probable that effects on T-cell responses cannot entirely explain increased susceptibility to infection in patients treated with drugs that inhibit calcineurin/NFAT binding. The NFAT pathway is remarkably active in the majority of innate cells after PRR activation. When exposed to microbes, innate cells deficient in NFAT signaling exhibit a radically different transcriptional profile and phenotype, which significantly alters the outcome of adaptive immunity (Figure 3). It is possible that these effects also contribute to the higher susceptibility to fungal and viral infections observed in CsA/tacrolimus-immunosuppressed patients.
NFAT signaling in innate immune cells must now be considered as a major influence on the efficacy of various therapies that are already being used in the clinic. It was recently shown that low-dose IL-2 was able to prevent GVHD in patients whoreceived HSC transplants.108 The low levels of IL-2 injected subcutaneously were sufficient to ameliorate symptoms of GVHD and were associated with the expansion of FoxP3+ Tregs. These data are consistent with the observation that GVHD prophylaxis with sirolimus, which inhibits T-cell functions in a calcineurin-independent manner, is more effective than calcineurin inhibition in reducing risk of GVHD.108 Furthermore, the local delivery of IL-2 was found to reduce the formation of atherosclerotic plaques in mice.109 Thus, activation of the transcription factor NFAT in myeloid cells leads to the profound modulation of innate immunity as well as influencing adaptive immune responses.
The outcome of NFAT signaling in myeloid cells can vary depending on the cell type and experimental conditions. Whether NFAT transcriptional activity is activating or inhibitory depends heavily on cofactors, such as AP1 (Fos/Jun), MEF2, and GATA. Hence, the balance of these cofactors present may determine the different outcomes observed in different cell types. In conclusion, it would seem that NFAT activation in DCs provides signals that diminish immune responses. Apart from regulation of immune functions in DCs, the cell cycle and survival of both macrophages and NK cells are strongly influenced by NFAT, which may further alter the outcome of innate immune responses. Further investigation will now be needed to determine whether the roles played by NFAT in myeloid hematopoiesis and cell differentiation are either tissue specific9,107 or broadly conserved between sites.103 The impact of inhibitory drugs may also depend on the tissue-specific attributes of NFAT signaling. Myeloid cells are now emerging as additional targets of calcineurin inhibitors, and the potential effects of these drugs on myeloid lineage development will need to be assessed in treated patients. Future investigations will provide a better understanding of immunosuppressive drug effects on innate immune responses and on NFAT-induced gene products that shape the outcome of adaptive immunity.
Acknowledgments
The authors thank Dr Neil McCarthy of Insight Editing London for critical review of the paper.
This work was supported by Biomedical Research Council, A*STAR, Singapore.
Authorship
Contribution: J.F. and T.Z. wrote the review and revised the figures; A.Y.W.W. prepared figures; A.M. assisted with manuscript writing and prepared the table; H.-B.Y. assisted with manuscript writing; and P.R.-C. wrote and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paola Ricciardi-Castagnoli, Singapore Immunology Network (SIgN), Agency for Science, Technology and Research (A*Star), 8A Biomedical Grove, no. 04-06 Immunos, Biopolis, Singapore 138648; e-mail: paola_castagnoli@immunol.a-star.edu.sg.