Abstract
The chemokine CCL3/MIP-1α is a risk factor in the outcome of multiple myeloma (MM), particularly in the development of osteolytic bone disease. This chemokine, highly overexpressed by MM cells, can signal mainly through 2 receptors, CCR1 and CCR5, only 1 of which (CCR1) is responsive to CCL3 in human and mouse osteoclast precursors. CCR1 activation leads to the formation of osteolytic lesions and facilitates tumor growth. Here we show that formation of mature osteoclasts is blocked by the highly potent and selective CCR1 antagonist CCX721, an analog of the clinical compound CCX354. We also show that doses of CCX721 selected to completely inhibit CCR1 produce a profound decrease in tumor burden and osteolytic damage in the murine 5TGM1 model of MM bone disease. Similar effects were observed when the antagonist was used prophylactically or therapeutically, with comparable efficacy to that of zoledronic acid. 5TGM1 cells were shown to express minimal levels of CCR1 while secreting high levels of CCL3, suggesting that the therapeutic effects of CCX721 result from CCR1 inhibition on non-MM cells, most likely osteoclasts and osteoclast precursors. These results provide a strong rationale for further development of CCR1 antagonists for the treatment of MM and associated osteolytic bone disease.
Introduction
Establishment of multiple myeloma (MM) in the bone marrow niche is highly dependent on bone resorption and proximity to active osteoclasts (OCs).1,2 OC and MM cells support and nourish each other in vitro and in vivo.1,3,4 MM-associated osteolytic bone disease (OBD), which affects > 80% of patients, results from heightened bone catabolism and decreased bone formation and is characterized by severe bone pain and high rates of fractures, greatly impacting their quality and length of life.5,6
The chemokine CCL3/MIP-1α is one of the most important OC-activating factors produced by MM cells and is generally thought to contribute significantly to MM-associated OBD.7 In cell culture, CCL3 is among the most consistently identified OC-activating factors produced by primary and immortalized MM cells.8 The extent of CCL3 secretion by MM cells has been correlated with the extent of lytic bone lesions in patients.9 Serum levels of CCL3 are elevated in newly diagnosed MM patients and correlate with the extent of bone disease, bone resorption, and disease prognosis.10 High levels of CCL3 in bone marrow also correlate with MM disease stage and activity.11-13 Other chemokines that have been implicated in the pathogenesis of MM include CCL5/RANTES, which, like CCL3, is a potent activator of chemokine CCR1 and CCR5 receptors.14
We and others have shown that the pathogenic interplay between MM cells and the bone marrow environment is mediated, in part, by a paracrine mechanism whereby CCL3, secreted by MM cells, stimulates OC activity.15 At the same time, CCL3 also inhibits osteoblast (OB) formation, further contributing to the imbalance between bone resorption and bone formation.16 On the other hand, measurements of CCR1 expression on MM cell lines and primary MM cells have been inconsistent from laboratory to laboratory.3,17
Even though MM has the highest incidence of OBD among all malignancies, OBD is also associated with metastases of solid tumors to the skeleton. In this setting, the clinical benefit associated with neutralization of key OC-activating factors, such as receptor activator of nuclear factor-κB ligand (RANKL) and IL-6, has been documented.18,19 Interestingly, despite the large body of literature on the potential role of CCL3 in MM and associated OBD, no therapies targeting CCL3 or its receptors have been evaluated clinically in the cancer/OBD setting. This paucity of clinical progress might in part be the result of the historical difficulty in developing chemokine-targeted drugs,20,21 or might be related to early reports suggesting that concurrent inhibition of both receptors (CCR1 and CCR5) through which CCL3 signals might be required to completely neutralize its OC-activating effects.22-24
In the present study, we challenge the published reports that CCR1 inhibition alone is insufficient to recapitulate the profound benefits seen with anti-CCL3 antibodies in preclinical MM models.15 Having recently shown that high levels of receptor inhibition are required to effectively block CCR1-mediated effects in preclinical models of inflammation and in rheumatoid arthritis patients,25 our analysis indicated that those earlier preclinical MM studies might not have achieved adequate circulating levels of the CCR1 antagonist. Thus, we have revisited this question using a novel, extremely potent and selective small-molecule CCR1 antagonist, CCX721.26,27 This orally bioavailable compound is a close chemical analog of CCX354, another CCR1 antagonist that recently showed clinical efficacy in rheumatoid arthritis.25,28 The potency and selectivity of CCX721 toward murine CCR1, as well as its pharmacokinetic (PK) and pharmacodynamic requirements for producing robust systemic CCR1 inhibition in rodents, were thoroughly evaluated, to select adequate dosing regimens to test the “anti-CCR1” hypothesis in the well-characterized murine 5TGM1-GFP model of MM/OBD.29,30 In addition to describing the profound effects of CCX721 on tumor burden and bone disease in this model in connection with either therapeutic or prophylactic intervention, we also describe its effects on human OC formation in vitro, which support the potential clinical evaluation of this or similar compounds against human MM.
Methods
Cells and reagents
Human monocytic THP-1 cells and multiple myeloma cell lines (RPMI8226, U266, MM1.S, and MM1.R) were from ATCC. Human monocytes were isolated from buffy coats (Stanford Blood Center) using MACS separation reagents (Miltenyi Biotec). Mouse and rat leukocytes were collected by peritoneal lavage 24 hours after injection of thioglycollate into the peritoneal cavity. CCX72126,27 was synthesized at ChemoCentryx. Zoledronic acid (Zometa; Novartis Pharmaceuticals) was obtained as a 5 mg/100 mL sterile solution. Bortezomib (Velcade) was from Biotang Inc. Recombinant chemokines and cytokines were from R&D Systems; the N-terminal truncated superagonist forms of CCL15/leukotactin and CCL23/CKβ8 were used in these studies. [125I]-CCL3 was obtained from PerkinElmer. The Ly-6G mAb was obtained from BD Biosciences. Human, rat, and mouse serum were obtained from Bioreclamation. The murine 5TGM1-GFP cell line and its routine maintenance were described previously.29,30
In vitro assays
Chemotaxis, cytoplasmic calcium release, and radioligand binding assays were conducted as previously described.25,31 The OC formation assays were conducted as described,3 using freshly isolated monocytes from human blood. The MTT cell proliferation assay (ATCC) was carried out per the manufacturer's instructions. Inhibition values (IC50) were calculated using nonlinear regression with a 1-site competition model (GraphPad Prism). A2 values were calculated from the following equation:
pA2 = p[CCX721] − p[(A′/A) − 1]
where A reflects the potency (EC50) of the agonist in the absence of antagonist and A′ reflects the potency of the agonist in the presence of antagonist at a given drug concentration of CCX721. Inhibition of CCR1 function on blood leukocytes was calculated using the potency of CCX721 in a chemotaxis assay and the plasma drug levels in the following equation:
% functional inhibition = 100 − (100/([CCX721]/A2 + 1))
where A2 is the value measured with chemotaxis assays conducted in serum and [CCX721] is the plasma concentration of CCX721.
Thioglycollate-induced peritonitis
Anesthetized Wistar rats (n = 6/group; The Jackson Laboratory) were injected intraperitoneally with thioglycollate and dosed orally with CCX721 or vehicle (sesame oil; Sigma-Aldrich; 2.5-mL/kg dose volume) every 12 hours, starting 2 hours before and ending 34 hours after thioglycollate injection. Peritoneal cells were collected via lavage 48 hours after thioglycollate injection, and the number of Ly-6G+ peritoneal cells was determined using flow cytometry. Satellite animals (n = 2) were used for PK measurements: peripheral blood, collected into EDTA tubes at indicated time points, was used to prepare plasma samples that were analyzed by HPLC/mass spectrometry.
Mouse PK studies in C57BL/6 mice
CCX721 (100 mg/kg) was dosed orally to 9 female C57BL/6 mice (The Jackson Laboratory) formulated in sesame oil at a concentration of 10 mg/mL. Blood samples were collected in EDTA tubes at predetermined time points after each dose, covering 0.25-, 0.5-, 1-, 2-, 4-, 6-, 8-, and 24-hour time points. Plasma samples were then prepared and analyzed by HPLC/mass spectrometry.
Murine 5TGM1-GFP myeloma model
Animal studies were conducted as previously described30 using 6- to 9-week-old female C57BL/KaLwRijHsd mice (Harlan Laboratories) in accordance with National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. All studies with mice were approved by the appropriate Animal Care and Use Committees. Myeloma lesions were induced in mice by intravenous inoculation of 106 viable 5TGM1-GFP cells through tail veins. Animals were randomized into groups and dosed twice daily by oral gavage with CCX721 (100 mg/kg) or vehicle (sesame oil, 2.5-mL/kg dose volume); alternatively, animals were dosed with zoledronic acid (120 μg/kg) by subcutaneous injection given twice weekly. In the therapeutic dosing protocol, myeloma localization to the bone was confirmed at day 18, using whole-body optical fluorescence imaging as described previously,30 and animals were then randomized into groups for treatment. Mouse serum from whole blood, collected by cardiac puncture at time of death, was used to assess 5TGM1-specific monoclonal paraprotein (IgG2bK) levels and was assayed by a specific in-house ELISA.32 After death, the skeleton was harvested and rapidly imaged for fluorescent tumor foci before significant effects from postmortem changes occurred. Bones were fixed in 10% formalin for 48 hours and subsequently decalcified in 14% EDTA for 1 to 2 weeks. Serial 5-μm-thick bone sections were stained with hematoxylin and eosin and for tartrate-resistant acid phosphatase (TRAP) activity, a marker of OC activity, and the number of OCs per bone surface determined, as described previously.15 Radiographs of fixed bones were obtained using Faxitron Radiographic Unit (Kodak) and quantitated using MetaMorph Version 6.1r6 imaging software (Molecular Devices) by investigators blinded to the experimental protocol. Plasmacytomas were established by injecting 5TGM1-GFP cells subcutaneously above the hind flanks of naive C57BL/kaLwRijHsd mice. Mice, inoculated with 107 cells, were randomly assigned to receive vehicle (sesame oil) or CCX721 twice daily by oral gavage for 21 days. Just before death, tumor diameters were measured in 3 dimensions with an electronic caliper and tumor volumes calculated; volume = 4/3π (½d1) × (½d2) × (½d3).
Data analysis
Statistical analyses using the Student t test or the nonparametric Mann-Whitney test, as indicated, were calculated with GraphPad Prism 4 Version 4.03 software.
Results
CCX721 is a potent and selective antagonist of human CCR1
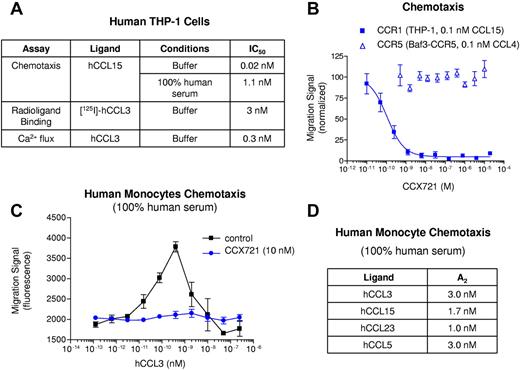
The small-molecule CCX721 was characterized extensively, using a variety of in vitro assays, to determine its potency and selectivity for human CCR1 versus other chemokine or otherwise pharmacologically relevant receptors (Figure 1; supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). CCX721 inhibited chemotaxis of human monocytic THP-1 cells toward the CCR1 chemokine CCL15 with a potency (IC50) of 0.02nM. When this assay was conducted in the presence of 100% human serum, which contains proteins, such as albumin, that reversibly bind small molecules, CCX721 retained its potent inhibition of CCR1-mediated chemotaxis, with an IC50 of 1.1nM (Figure 1A). In contrast, CCX721 did not inhibit CCR5-mediated chemotaxis, even when CCX721 was present at a high concentration (20μM; Figure 1B), indicating greater than 106-fold selectivity for CCR1 relative to CCR5. CCX721 potently inhibited [125I]-CCL3 binding and CCL3-induced calcium release in THP-1 cells with IC50 values of 3 and 0.3nM, respectively (Figure 1A). No CCX721-mediated inhibition of non-CCR1 chemokine receptors was observed either on primary human blood monocytes or cultured lymphocytes (supplemental Figure 1) or using recombinant receptors on transfected cell lines (supplemental Table 1). Similarly, no activity was identified against a panel of 55 pharmacologically important targets (supplemental Table 1). CCX721 retained its potency when tested using human blood monocytes; 10nM CCX721 fully inhibited the chemotaxis of these cells toward CCL3, even when the assay was conducted in 100% human serum (Figure 1C); addition of CCX721 at concentrations ≥ 1000-fold higher than this did not affect monocyte chemotaxis toward other chemotactic proteins (CCL2/MCP-1, CCL8/MCP-2, and CCL13/MCP-4; data not shown), confirming its selectivity for CCR1. The potency of CCX721 toward human CCR1 (Kd = 0.17nM) was confirmed using [125I]-CCL15 saturation binding studies on freshly isolated human monocytes (supplemental Figure 2). CCX721 was also shown to inhibit, in a fairly equipotent manner, the chemotaxis and intracellular calcium release of human monocytes in response to the 4 main CCR1 chemokines (CCL3, CCL5, CCL15, and CCL23; Figure 1D; supplemental Figure 3).
CCX721 is a potent antagonist of CCR1-mediated functions, as tested with human monocytes, with all known chemokine ligands and in physiologically relevant human serum. (A) Potency values for CCX721 are shown, measured using CCR1-expressing THP-1 cells, and include results with chemotaxis, radioligand binding, and cytoplasmic calcium flux assays. (B) CCR1- or CCR5-specific chemotaxis assays, with increasing concentrations of CCX721 added, were conducted in chemotaxis buffer with n = 8 replicates per point; migration signals were normalized to controls. (C) Chemotaxis of freshly isolated human monocytes to a range of CCL3 concentrations, with n = 8 replicates per point, was conducted in the presence of human serum, with or without the addition of 10nM CCX721. (D) Summary data for all CCR1 ligands in this serum chemotaxis assay.
CCX721 is a potent antagonist of CCR1-mediated functions, as tested with human monocytes, with all known chemokine ligands and in physiologically relevant human serum. (A) Potency values for CCX721 are shown, measured using CCR1-expressing THP-1 cells, and include results with chemotaxis, radioligand binding, and cytoplasmic calcium flux assays. (B) CCR1- or CCR5-specific chemotaxis assays, with increasing concentrations of CCX721 added, were conducted in chemotaxis buffer with n = 8 replicates per point; migration signals were normalized to controls. (C) Chemotaxis of freshly isolated human monocytes to a range of CCL3 concentrations, with n = 8 replicates per point, was conducted in the presence of human serum, with or without the addition of 10nM CCX721. (D) Summary data for all CCR1 ligands in this serum chemotaxis assay.
Identification of an oral dosing regimen of CCX721 that is well tolerated and provides effective systemic CCR1 blockade in rodents
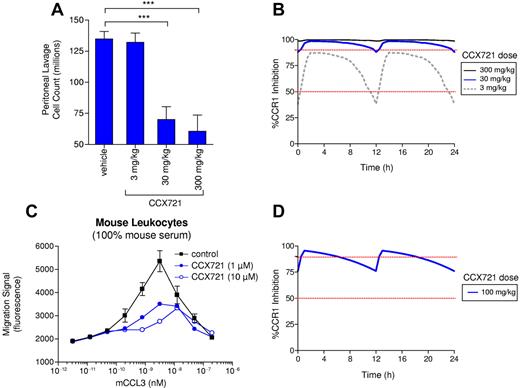
Three CCX721 oral dosing regimens were tested in a rat model (sterile peritonitis) of inflammation and leukocyte recruitment. At 2 of these doses (30 and 300 mg/kg), there was significant inhibition of peritoneal leukocyte recruitment (Figure 2A). The extent of CCR1 inhibition on blood leukocytes achieved in this study was estimated from the PK profile of CCX721 and its potency on rat CCR1 (A2 = 0.1μM; supplemental Figure 4). The 2 effective dosing regimens produced plasma concentrations of CCX721 that block ≥ 90% of blood leukocyte CCR1 almost the entire time (Figure 2B), whereas the relatively ineffective 3-mg/kg doses resulted in only modest coverage (40%-85% CCR1 inhibition throughout the day). The potency (A2 = 0.2μM) of CCX721 against mouse CCR1 was determined in the same manner (Figure 2C), and this potency, together with its PK profile in mice, was used to estimate that CCR1 coverage would range from 76% to 96% throughout the day, after 100 mg/kg twice daily oral dosing in mice (Figure 2D). CCX721 does not inhibit mouse CCR5, nor does it inhibit other pharmacologically relevant targets that might confuse the interpretation of any pharmacology studies (supple-mental Figure 5; supplemental Table 1). In addition, this dose (100 mg/kg) was well tolerated by rats for 7 consecutive days (data not shown). On this basis, 100 mg/kg CCX721, administered by oral gavage twice daily, was selected for the MM mouse studies as a dosing regimen that is well tolerated, provides excellent coverage of CCR1 in vivo, and is free of any activity on other chemokine receptors. Given that the potency of CCX721 on human CCR1 is ∼ 200 times greater than for mouse or rat CCR1, the human dose equivalent to that used in the mouse studies would be significantly lower.
Sustained CCR1 blockade correlates with an effective anti-inflammatory response in a rodent thioglycollate-induced peritonitis assay and coverage levels achieved in the mouse myeloma assay. (A) CCX721 was administered in oral dosages of 3, 30, or 300 mg/kg twice daily in a rat thioglycollate-induced peritonitis assay, using cell count in the peritoneal lavage as the primary readout. (B) Functional inhibition of rat CCR1 by CCX721 over a 24-hour period, calculated from the PK measurements taken in satellite animals and in vitro CCX721 dose-response inhibition of CCR1-mediated leukocytes in 100% rat serum. (C) Chemotaxis of mouse thioglycollate-elicited leukocytes toward mouse CCL3 in 100% mouse serum and in the presence of various concentrations of CCX721 (n = 8 replicates per point, assay repeated 3 times). (D) Functional inhibition of mouse CCR1 by a 100-mg/kg orally administered dose of CCX721 given twice daily over a 24-hour period, calculated from the PK measurements taken in satellite animals and in vitro CCX721 dose-response inhibition of CCR1-mediated leukocytes in 100% mouse serum. ***P < .001 (Student t test).
Sustained CCR1 blockade correlates with an effective anti-inflammatory response in a rodent thioglycollate-induced peritonitis assay and coverage levels achieved in the mouse myeloma assay. (A) CCX721 was administered in oral dosages of 3, 30, or 300 mg/kg twice daily in a rat thioglycollate-induced peritonitis assay, using cell count in the peritoneal lavage as the primary readout. (B) Functional inhibition of rat CCR1 by CCX721 over a 24-hour period, calculated from the PK measurements taken in satellite animals and in vitro CCX721 dose-response inhibition of CCR1-mediated leukocytes in 100% rat serum. (C) Chemotaxis of mouse thioglycollate-elicited leukocytes toward mouse CCL3 in 100% mouse serum and in the presence of various concentrations of CCX721 (n = 8 replicates per point, assay repeated 3 times). (D) Functional inhibition of mouse CCR1 by a 100-mg/kg orally administered dose of CCX721 given twice daily over a 24-hour period, calculated from the PK measurements taken in satellite animals and in vitro CCX721 dose-response inhibition of CCR1-mediated leukocytes in 100% mouse serum. ***P < .001 (Student t test).
Human peripheral blood monocytes and mouse bone marrow monocytes express functional CCR1 receptor
Even though some authors have questioned, based on genetic manipulation of CCR1, the presence of functional receptor on mouse monocytes,33 freshly isolated blood monocytes from human donors (supplemental Figure 1) and CD14+-enriched bone marrow monocytes (OC precursors) from C57Bl/6 mice (supplemental Figure 5A) respond (by intracellular calcium release) to stimulation by CCR1 chemokines, an effect that can be completely blocked by CCX721. Expression of mRNA for several chemokine receptors (CCR1, CCR2, CCR3, and CCR5) was observed in mouse bone marrow monocytes (supplemental Figure 6). However, only CCR1 stimulation produced a functional response (calcium release); of note, CCR5 stimulation with CCL4/MIP-1β, a mCCR5-active chemokine, failed to induce a calcium response (supplemental Figure 5A).
Prophylactic administration of CCX721 in the 5TGM1 immunocompetent model of disseminated MM results in a greatly reduced tumor burden and fewer myeloma-triggered OCs
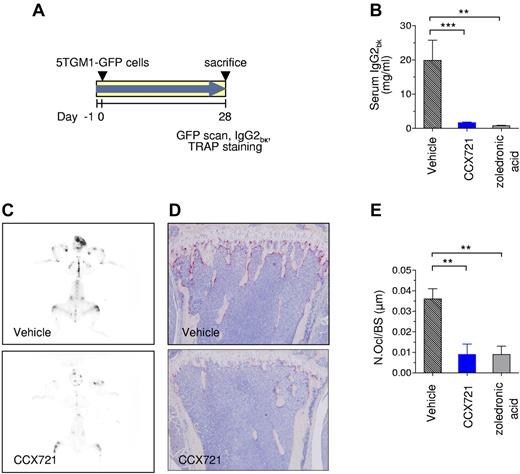
The 5TGM1 MM model is a well-established preclinical model shown to be predictive of clinical efficacy. The expression of chemokine receptors by the myeloma 5TGM1-GFP cell line was characterized extensively before use. Although CCR1 mRNA was detected by RT-PCR (data not shown), these cells did not express CCR1 protein as assessed either by a functional assay (supplemental Figure 7) or [125I]-CCL3 binding (data not shown). Consistent with the characterization of other MM cells (cultured or primary isolates),14 the 5TGM1-GFP cells secreted high levels of CCL3 in culture, as determined by ELISA (∼ 250 pg/mL from 48-hour cultures). Naive C57BL/KaLwRijHsd mice were randomized into 3 groups: group 1, CCX721 (100 mg/kg, orally, twice daily; n = 12 mice); group 2, vehicle (100% sesame oil, orally, twice daily; n = 12 mice); and group 3, zoledronic acid (0.12 mg/kg, subcutaneously, twice weekly; n = 5 mice). After 1 day of drug treatment (study day 0), the mice were injected intravenously with syngeneic 5TGM1-GFP cells (Figure 3A), and the study continued for the designated 4-week duration. After 28 days, serum monoclonal paraprotein levels (IgG2bκ, biomarker of tumor burden) were reduced from 19.9 ± 5.9 mg/mL for the vehicle control to 1.7 ± 0.2 mg/mL for the mice treated with CCX721 (Figure 3B; P < .001), an effect comparable with that achieved with the positive control, zoledronic acid (0.8 ± 0.1 mg/mL; P < .01). No difference in normalized spleen weight was observed (supplemental Figure 8A). The reduction in overall tumor burden was consistent with the attenuation of tumor-associated green fluorescence on whole skeleton fluorescent scans (Figure 3C). In addition, the number of TRAP+ OCs lining bone surfaces was markedly reduced in CCX721-treated mice compared with vehicle-treated controls, as seen in the representative histology images (Figure 3D). The number of OCs per bone surface (μm) was reduced from 0.036 ± 0.005 in vehicle control animals to 0.009 ± 0.005 in CCX721-treated animals (Figure 3E; P < .01), a reduction comparable with that in zoledronic acid-treated animals. The size of osteolytic bone lesions measured from radiographs of the humerus trended to improvement with treatment by CCX721 or zoledronic acid, but the improvement did not achieve statistical significance with this group size (supplemental Figure 8B-C).
Prophylactic dosing with CCX721 reduces both myeloma tumor burden and myeloma-triggered OC formation. (A) Schematic diagram of the 28-day study with naive syngeneic female C57BL/KaLwRijHsd mice that were inoculated intravenously with 5TGM1-GFP MM cells, randomized into groups, and dosed twice daily by oral gavage with either vehicle or CCX721 (100 mg/kg); an additional control group received zoledronic acid (120 μg/kg) by subcutaneous injection twice weekly. (B) Serum monoclonal IgG2bK paraprotein titers from samples taken on day 28 after tumor cell inoculation. (C) Representative whole-skeleton fluorescence scans detecting fluorescent myeloma foci in bone (image negatives shown; see supplemental Figure 10 for green fluorescence color originals). (D) OC activity assessed by TRAP staining (red) in the long bones of tumor-bearing mice, representative images using an Olympus BX41 microscope (10× magnification). (E) Histomorphometric analysis of the number of OCs per bone surface. **P < .01 (Mann-Whitney test); ***P < .001 (Mann-Whitney test).
Prophylactic dosing with CCX721 reduces both myeloma tumor burden and myeloma-triggered OC formation. (A) Schematic diagram of the 28-day study with naive syngeneic female C57BL/KaLwRijHsd mice that were inoculated intravenously with 5TGM1-GFP MM cells, randomized into groups, and dosed twice daily by oral gavage with either vehicle or CCX721 (100 mg/kg); an additional control group received zoledronic acid (120 μg/kg) by subcutaneous injection twice weekly. (B) Serum monoclonal IgG2bK paraprotein titers from samples taken on day 28 after tumor cell inoculation. (C) Representative whole-skeleton fluorescence scans detecting fluorescent myeloma foci in bone (image negatives shown; see supplemental Figure 10 for green fluorescence color originals). (D) OC activity assessed by TRAP staining (red) in the long bones of tumor-bearing mice, representative images using an Olympus BX41 microscope (10× magnification). (E) Histomorphometric analysis of the number of OCs per bone surface. **P < .01 (Mann-Whitney test); ***P < .001 (Mann-Whitney test).
Therapeutic CCX721 treatment of mice with established skeletal 5TGM1 myeloma lesions markedly reduced tumor burden
Eighteen days after being injected intravenously with 5TGM1-GFP cells (Figure 4A), C57BL/KaLwRijHsd mice were imaged by noninvasive whole-body optical fluorescence scanning. Mice in which established (GFP+) myeloma lesions in the skeleton were confirmed were then randomized into treatment groups: CCX721 (100 mg/kg; n = 9 mice) or vehicle (sesame oil; n = 5 mice) orally, twice daily; or zoledronic acid (0.12 mg/kg; n = 5 mice; subcutaneously, twice weekly) and dosed for 10 consecutive days. At the end of the study, serum monoclonal IgG2bκ paraprotein levels were reduced from 6.0 ± 0.6 mg/mL (vehicle control) to 1.9 ± 0.2 mg/mL (CCX721 treatment; Figure 4B; P < .001), an effect comparable with that achieved with the positive control, zoledronic acid (1.7 ± 0.1 mg/mL; P < .01). There were no significant differences in serum IgG2bκ levels among the 3 groups at the beginning of dosing on day 18 (Figure 4C). By day 28, overall tumor burden was reduced in the CCX721 group, as shown by reduced serum IgG2bκ titers (Figure 4B-C) and tumor-associated green fluorescence on whole skeleton scans (Figure 4D top panels); no difference in normalized spleen weight was observed (Figure 5D; supplemental Figure 9A). The number of TRAP+ OCs lining bone surfaces was reduced in CCX721-treated mice compared with vehicle-treated controls, as seen in the representative histology images (Figure 4D bottom panels). The number of OCs per bone surface (μm) was reduced from 0.066 ± 0.002 in vehicle control animals to 0.046 ± 0.003 in CCX721-treated animals (Figure 4E; P < .01). Consistent with this, the size of osteolytic bone lesions was reduced with treatment with either CCX721 or zoledronic acid, although this did not reach statistical significance (supplemental Figure 9B-C).
Therapeutic dosing with CCX721 reduces myeloma tumor burden. (A) Schematic diagram of the 28-day study with naive syngeneic female C57BL/KaLwRijHsd mice; 18 days after intravenous inoculation with 5TGM-GFP MM cells, establishment of tumors was confirmed by whole-body optical fluorescence imaging, and the mice were then randomized into groups. The groups received either vehicle or CCX721 (100 mg/kg) dosed orally twice a day beginning on day 18; an additional control group received zoledronic acid (120 μg/kg) by subcutaneous injection twice weekly. (B) Serum monoclonal IgG2bK paraprotein titers from samples taken on day 28 after tumor cell inoculation. (C) Serum IgG2bK titers from samples taken on days −1, 18, and 28 (mean ± SEM). (D) Representative whole-skeleton fluorescence scans detecting fluorescent myeloma foci in bone, taken on day 28 images (image negatives shown; see supplemental Figure 10 for color originals). OC activity assessed by TRAP staining (red) in the long bones of tumor-bearing mice, representative images using an Olympus BX41 microscope (10× magnification). (E) Histomorphometric analysis of the number of OCs per bone surface. **P < .01 (Mann-Whitney test); ***P < .001 (Mann-Whitney test).
Therapeutic dosing with CCX721 reduces myeloma tumor burden. (A) Schematic diagram of the 28-day study with naive syngeneic female C57BL/KaLwRijHsd mice; 18 days after intravenous inoculation with 5TGM-GFP MM cells, establishment of tumors was confirmed by whole-body optical fluorescence imaging, and the mice were then randomized into groups. The groups received either vehicle or CCX721 (100 mg/kg) dosed orally twice a day beginning on day 18; an additional control group received zoledronic acid (120 μg/kg) by subcutaneous injection twice weekly. (B) Serum monoclonal IgG2bK paraprotein titers from samples taken on day 28 after tumor cell inoculation. (C) Serum IgG2bK titers from samples taken on days −1, 18, and 28 (mean ± SEM). (D) Representative whole-skeleton fluorescence scans detecting fluorescent myeloma foci in bone, taken on day 28 images (image negatives shown; see supplemental Figure 10 for color originals). OC activity assessed by TRAP staining (red) in the long bones of tumor-bearing mice, representative images using an Olympus BX41 microscope (10× magnification). (E) Histomorphometric analysis of the number of OCs per bone surface. **P < .01 (Mann-Whitney test); ***P < .001 (Mann-Whitney test).
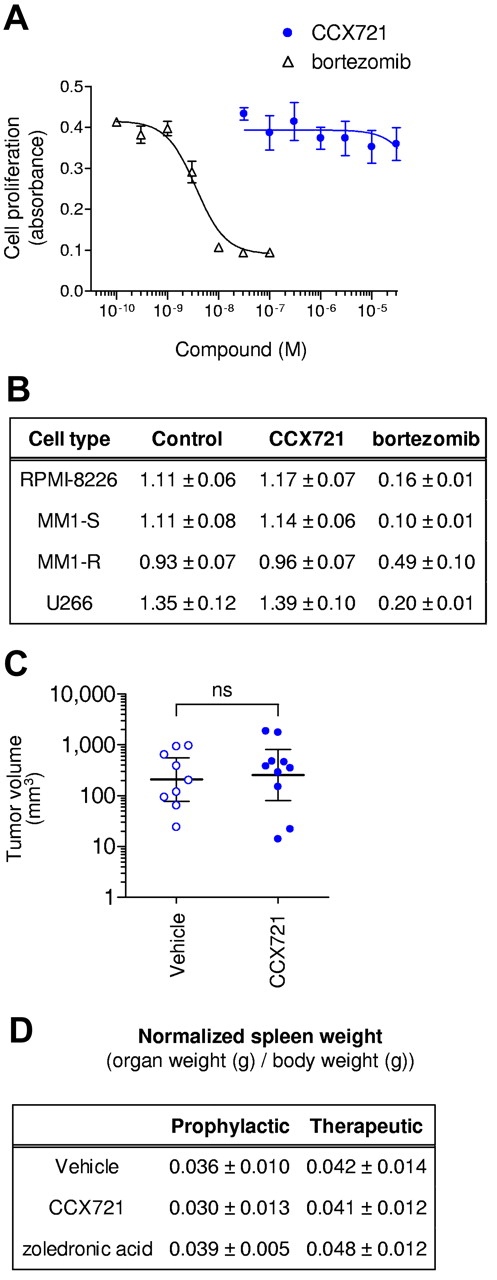
CCX721 does not reduce myeloma tumor burden through direct killing of myeloma cells. (A) MTT cellular proliferation assay of 5TGM1-GFP cells after overnight growth in the presence of increasing concentrations of CCX721 or the proteasome inhibitor bortezomib (n = 8 replicates per point, assay repeated n = 3 times). (B) Table of MTT cellular proliferation assay results (absorbance, mean ± SD) with cultured human myeloma cells grown for 3 days in the presence of CCX721 (1000nM) or bortezomib (10nM; n = 8 replicates per point, assay repeated n = 3 times). CCX721 data were not significantly different (P > .05) from control, but bortezomib data were (P < .05). (C) Plasmacytoma volume measured 21 days after 107 5TGM1-GFP cells were inoculated subcutaneously in naive C57BL/kaLwRijHsd mice. These mice were randomly assigned to receive vehicle (sesame oil) or CCX721 (100 mg/kg) twice daily by oral gavage for 21 days. Plasma levels of CCX721 were consistent with previous studies. ns indicates not significant. (D) Table of spleen weights (normalized to body weight) for the 2 dosing regimens; data were not significantly different from control (P > .05).
CCX721 does not reduce myeloma tumor burden through direct killing of myeloma cells. (A) MTT cellular proliferation assay of 5TGM1-GFP cells after overnight growth in the presence of increasing concentrations of CCX721 or the proteasome inhibitor bortezomib (n = 8 replicates per point, assay repeated n = 3 times). (B) Table of MTT cellular proliferation assay results (absorbance, mean ± SD) with cultured human myeloma cells grown for 3 days in the presence of CCX721 (1000nM) or bortezomib (10nM; n = 8 replicates per point, assay repeated n = 3 times). CCX721 data were not significantly different (P > .05) from control, but bortezomib data were (P < .05). (C) Plasmacytoma volume measured 21 days after 107 5TGM1-GFP cells were inoculated subcutaneously in naive C57BL/kaLwRijHsd mice. These mice were randomly assigned to receive vehicle (sesame oil) or CCX721 (100 mg/kg) twice daily by oral gavage for 21 days. Plasma levels of CCX721 were consistent with previous studies. ns indicates not significant. (D) Table of spleen weights (normalized to body weight) for the 2 dosing regimens; data were not significantly different from control (P > .05).
CCR1 blockade does not have a direct effect on MM cell growth outside the bone marrow microenvironment
Overnight exposure of 5TGM1-GFP cells to CCX721, at concentrations up to 30μM, had no effect on cellular proliferation, as measured with an MTT assay (CC50 > 30μM; Figure 5A). In contrast, the positive control bortezomib was very cytotoxic (CC50 = 3nM). Similar results were obtained with human myeloma RPMI8226, U266, MM1.S, and MM1.R cells (Figure 5B). Likewise, when C57BL/kaLwRijHsd mice were inoculated with 107 5TGM1-GFP cells under the skin (Figure 5C), the CCX721 dosing regimen used previously (100 mg/kg, orally, twice daily; n = 10 mice) had no effect on the development or progression of subcutaneous plasmacytomas, as assessed by tumor volumes measured after 21 days, compared with vehicle-treated mice (100% sesame oil, orally, twice daily; n = 9 mice). In the same experiment, administration of bortezomib (0.5 mg/kg, intraperitoneally, 3 times per week) almost completely abrogated plasmacytoma growth.
CCR1 blockade with CCX721 reduces in vitro OC formation
Peripheral blood mononuclear cells from healthy human donors were cultured in the presence of M-CSF and RANKL, as described,3 with or without CCX721 (10nM) added twice weekly. After 21 days in culture, multinucleated OCs had formed in the control group, with many of the OCs containing 20 or more nuclei (Figure 6A); in contrast, significantly fewer OCs formed in the presence of CCX721, and these were much smaller in size than those in the vehicle-treated group. Importantly, enumeration of the TRAP+ OCs, defined as staining positive for TRAP and having ≥ 3 nuclei, indicated that CCR1 inhibition significantly reduced the number of multinucleated OCs formed under these conditions (Figure 6B; P < .05).
CCR1 blockade inhibits formation of mature OCs. (A) Representative images of monocyte cultures, grown in the presence of added RANKL and M-CSF, forming mature OCs, as shown by TRAP staining (red). Cultures were grown in the presence or absence of CCX721 (10nM). Images taken with Nikon Diaphot T20 (400× magnification). (B) Number of TRAP-positive multinucleated cells as a percentage of total cells cultured (representative results shown, experiment conducted n = 3 times). *P < .05 (Student t test).
CCR1 blockade inhibits formation of mature OCs. (A) Representative images of monocyte cultures, grown in the presence of added RANKL and M-CSF, forming mature OCs, as shown by TRAP staining (red). Cultures were grown in the presence or absence of CCX721 (10nM). Images taken with Nikon Diaphot T20 (400× magnification). (B) Number of TRAP-positive multinucleated cells as a percentage of total cells cultured (representative results shown, experiment conducted n = 3 times). *P < .05 (Student t test).
Discussion
A large body of literature indicates that CCL3 production by MM cells controls the extent of bone lesion and tumor burden in mouse models of MM.34 We have previously shown that antibody neutralization of CCL3 prevents osteolysis and tumor growth in a spontaneous MM model in mice.15 Thus, MM cells are capable of hijacking signaling pathways (eg, RANKL, in addition to CCL3),35 which under nonpathogenic conditions are involved in the regulation of bone function, to promote their establishment and proliferation in the BM environment; the resulting stimulation of OC formation and inhibition of OB activity account for the resulting OBD.3,16
The mechanism by which CCL3 elicits these effects involves activation of its cognate receptor(s) on OC precursors (OCPs) and/or mature OCs, as well as OBs. OCPs, OCs, and OBs have been shown to express mRNA for the 2 main CCL3 receptors, CCR1 and CCR5.16,36-38 Both receptors have been claimed to play a role in mouse OC differentiation and bone resorption capacity of CCL3 in vitro.22,23 We detected small amounts of CCR5 mRNA in mouse bone marrow CD14+ mononuclear cells and human peripheral blood CD14+ monocytic cells; however, there was no CCR5-mediated functional response in either cell population. On the other hand, CCR1 mRNA and CCR1-mediated calcium flux, sensitive to inhibition by the selective CCR1 antagonist CCX721, were observed in both cell types. Expression of CCR1 on mouse monocytes has been questioned33 ; however, our results are consistent with the reported in vitro chemotaxis of mouse OCP toward several major CCR1 chemokines (CCL3, CCL5, and CCL23)39 and the presence of CCR1 mRNA in mouse bone marrow cells, which increased in response to M-CSF/RANKL stimulation.37 CCR1-activating chemokines have been identified as potential “bone-coupling factors,“ which mediate the crosstalk between OCs and OBs to maintain bone remodeling.40 Genetic deletion of CCR1 in mice results in disruption of bone catabolic and anabolic activities, characterized by impaired osteoclastogenesis and reduced RANKL expression by OBs.40 A recent report indicated that CCR1 activation by CCL3 is important for the M-CSF/RANKL-stimulated differentiation of human OCP into multinucleated TRAP+ OCs.3 We have confirmed these observations using the CCR1 antagonist CCX721. The direct contribution of CCR5 to OC maturation is less clear. In our hands, MK-0812, a potent dual CCR2/CCR5 antagonist,20 had no effects on human OCP maturation (data not shown), suggesting that, under the conditions of this assay, neither CCR2 nor CCR5 plays a role in OC maturation. Thus, our results indicate that CCR1 is the key CCL3 receptor expressed on the human and mouse monocytic cells that give rise to mature OCs.
Separate evaluation of CCR1 and CCR5 antagonists in a mouse model of MM bone disease indicated that therapeutic treatment (after disease establishment) with either type of antagonist had robust effects on a number of parameters, including bone lesions, trabecular bone area, and bone microvessel density; however, only the CCR1 antagonist was able to impact tumor load.22 These observations led these investigators to conclude that concurrent blockade of CCR1 and CCR5 might be required to mimic the degree of efficacy noted earlier after neutralization of CCL3. We decided to revisit this conclusion because of the lack of evidence that this early work accomplished sufficiently high levels of chemokine receptor inhibition in vivo. The CCR1 antagonist used in such early work may have lacked adequate potency, selectivity, and/or PKs or was not adequately dosed to fully explore the therapeutic potential associated with CCR1 inhibition.21 On the other hand, the selectivity of the CCR5 antagonist used in that study, a compound with well-known anti-HIV activity, has only been evaluated against a small number of other chemokine receptors, which identified potent inhibition of CCR2 and leaves open the possibility of some additional non-CCR5 activity.41 CCX721, the compound used in our studies, was shown to possess an affinity for human CCR1 of ∼ 0.02nM (in buffer), excellent selectivity, and oral bioavailability. The optimal dosing regimen and tolerability of this molecule (100 mg/kg twice daily) were determined in rodents (full prevention in a sterile peritonitis model) before conducting an assessment of its effects on MM tumor growth and associated OBD in the well-established mouse 5TGM1 model.
The 5TGM1 mouse MM model was described more than a decade ago. 5TGM1 cells, propagated in culture from a spontaneous mouse MM and injected into naive syngeneic C57BL/KaLwRij mice, induce myeloma disease, which exhibits similar features to human MM, such as osteolysis.42 Expression of GFP on these cells was introduced by one of us as a noninvasive way for in vivo detection of skeletal invasion by MM cells.30 The 5TGM1 model has been used to characterize the efficacy of ibandronate (Bondronat), inhibitors of RANKL, an anti-CCL3 antibody, a CD137 agonistic antibody, skeletally targeted radiotherapy, idiotype-specific T cells, the clinically proven proteasome inhibitor bortezomib (Velcade), and integrin inhibitors.15,29,30,32,43,44
In our first study, CCX721 dosing was initiated 1 day before inoculation with the 5TGM1-GFP cells, and it was continued for 28 days until animal death. Serum levels of the monoclonal paraprotein (IgG2bκ in this case) produced by the MM cells are correlated with the extent of tumor burden.15,30 We saw a pronounced (∼ 90%) and highly significant reduction in tumor burden in connection with CCR1 inhibition, a much larger effect than that previously described with either a CCR1 or CCR5 antagonist.22 Although we did not quantify the extent of green fluorescence in the skeleton of the mice, visual inspection of all the images confirmed the profound effect of the CCR1 antagonist on tumor burden. Concurrent with the benefit on tumor size, we also saw a pronounced (∼ 75%) decrease in the number of activated (TRAP+) OC lining bone surfaces, indicative of a profound effect on the process of MM-associated bone lysis. Particularly noteworthy is that the magnitude of the benefits associated with CCX721 treatment was indistinguishable from that obtained with the positive control zoledronic acid, a compound with well-documented antitumor and skeletal protective effects in MM and which has been in clinical use for years, despite significant safety and convenience issues.45,46
The relevance to human disease of prophylactic intervention in pharmacology studies is often questioned. This criticism is particularly relevant in this case because we could not entirely rule out a role of CCR1 in homing of the inoculated MM cells to the bone marrow. Our functional characterization in vitro of these cells indicated that such homing would most likely be mediated by CXCR4, an observation consistent with the high levels of its ligand (CXCL12/SDF-1) in bone marrow. Regardless of this, we decided to confirm the effects of CCX721 in the 5TGM1-GFP model in an interventional mode with compound dosing initiated after confirmed establishment of the tumor in mice. In this second study, our analysis confirmed the antimyeloma efficacy of CCX721 described earlier in the prophylactic experiment. As before, we also demonstrated that the CCR1 inhibitor was as effective as zoledronic acid at reducing serum levels of IgG2bκ paraprotein and intensity of green fluorescence in the whole-body bone scans.
There is increasing evidence that the tumor microenvironment in bone plays a role in MM progression, and the reciprocal feedback between tumor cells and the bone microenvironment has been referred to as a “vicious cycle.”47 Agents that exert an antitumor effect on MM in vivo may do so either by direct killing of MM cells or by modulating the microenvironment. However, several lines of evidence indicate that CCX721 does not reduce MM tumor burden via direct cytotoxicity to the tumor cells: (1) MM cells do not express functional CCR1; (2) CCX721 is not cytotoxic to human or mouse MM cells; (3) in contrast to bortezomib, which kills MM cells, CCX721 had no effect on subcutaneous 5TGM1 plasmacytoma development or progression; and (4) in the disseminated 5TGM1 MM model, although skeletal tumor burden was decreased, there was no effect on extraosseous (splenic) tumor burden as would be expected if CCX721 was cytotoxic to tumor cells. On the other hand, our data clearly establish that CCX721 treatment with CCX721 reduces OC numbers and osteolysis. Importantly, OC-MM interactions have been described to be a critical component of the vicious cycle.48 Taken together, these observations rule out a direct effect of CCX721 on MM cells and implicate an indirect effect via changes in the tumor microenvironment (Figure 7).
CCL3/CCR1 participates in the vicious cycle that allows myeloma cells to establish themselves in bone. CCR1 inhibition can break this cycle at multiple points. Expression of RANKL on bone marrow stromal cells (SCs) and OBs stimulates OC maturation (a). CCL3 is one of several key OC-activating factors produced by MM cells (b). MM-produced CCL3 leads to up-regulation of RANKL on SCs and OBs and concomitantly inhibits OB formation (c). Activated OCs enhance MM cell survival via direct integrin-mediated cell-cell interactions and by production of soluble factors, such as CCL3 (d). OC precursors and mature OCs express high levels of CCL3, which stimulates OC maturation via a CCR1-dependent pathway (e). CCR1 is expressed on many cell types in this cycle, including OC precursors, activated OCs, OBs, and, in some cases, MM cells; inhibition by CCR1 antagonists has the potential to break this pathogenic cycle at multiple points.
CCL3/CCR1 participates in the vicious cycle that allows myeloma cells to establish themselves in bone. CCR1 inhibition can break this cycle at multiple points. Expression of RANKL on bone marrow stromal cells (SCs) and OBs stimulates OC maturation (a). CCL3 is one of several key OC-activating factors produced by MM cells (b). MM-produced CCL3 leads to up-regulation of RANKL on SCs and OBs and concomitantly inhibits OB formation (c). Activated OCs enhance MM cell survival via direct integrin-mediated cell-cell interactions and by production of soluble factors, such as CCL3 (d). OC precursors and mature OCs express high levels of CCL3, which stimulates OC maturation via a CCR1-dependent pathway (e). CCR1 is expressed on many cell types in this cycle, including OC precursors, activated OCs, OBs, and, in some cases, MM cells; inhibition by CCR1 antagonists has the potential to break this pathogenic cycle at multiple points.
In summary, the magnitude and consistency of the benefits seen in our studies on MM tumor burden and bone catabolism stand in contrast to the much more modest effects seen earlier with another CCR1 antagonist.22 Given that the potency, selectivity, and dosing protocol for CCX721 have been optimized to ensure selective and complete CCR1 inhibition in vivo, we think that our studies best demonstrate the therapeutic potential of CCR1 inhibition in the MM/OBD setting.
These results do not rule out the possible contribution of CCR5-mediated effects in the pathology of MM. Although we could not detect functional CCR5 protein on OCP, CCR5 expression on bone marrow stromal cells and its regulation of RANKL expression on these cells have been described.24 In any case, even allowing for the possibility of CCR5 inhibition as a potential therapeutic approach in MM, our data clearly demonstrate the profound benefit that can be derived from intense CCR1 inhibition, even in established MM tumors.
Although our discussion of the mechanism of action of CCX721 in a mouse model of MM/OBD has focused largely on the bone catabolic effects of CCR1 activation, a number of other equally important biologic roles have been described for CCR1, including activation of OBs/RANKL, a direct proliferative effect on MM cells, modulation of the immune response to the tumor, angiogenesis, and hematopoiesis.8,16 Any of these could be important to the pathogenesis of MM and associated OBD.
Our study adds further weight to the growing realization that CCR1 inhibition is an attractive approach to the treatment of MM and associated OBD (Figure 7).8,49 In addition, CCR1 and some of its chemokine ligands have also been implicated in bone metastasis of certain solid cancers, such as renal cell carcinoma.49 This suggests the possibility of therapeutic use of a CCR1 antagonist in a variety of cancers and associated bone complications. The compound used in our present studies, CCX721, is a mouse-active structural analog of CCX354, a CCR1 antagonist that has recently shown positive results in a rheumatoid arthritis phase 2 clinical trial28 and is currently the most advanced clinical-stage CCR1 antagonist in clinical development.21,49 The present study provides the rationale for advancing one of these molecules, alone or in combination with existing FDA-approved agents, into the oncology space.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Mark Penfold for assistance with the gene expression studies and Ramaswamy Sharma and Paul Williams (University of Texas Health Science Center at San Antonio) for help with preparation and blinded analyses of the mouse radiographs and histology sections.
This work was supported in part by ChemoCentryx Inc (D.J.D., Y.W., L.C.S., J.P.P., S.M., Y.Z., P.Z., T.J.S., and J.C.J.). B.O.O. was supported by the National Institutes of Health/National Cancer Institute (K01CA104180 and P01CA040035) and ChemoCentryx (research grant). The University of Texas Health Science Center at San Antonio Flow Cytometry Core Facility is supported by the National Cancer Institute (Cancer Center Grant P30 CA054174).
National Institutes of Health
Authorship
Contribution: D.J.D., B.O.O., T.J.S., and J.C.J. designed the experiments and wrote the manuscript; B.O.O., A.G., and B.M. conducted the mouse myeloma studies; D.J.D. and S.M. performed the receptor coverage analysis; D.J.D., L.C.S., and Y.W. performed the experiments that define the potency and selectivity of CCX721; L.C.S. conducted the OC differentiation assays; and J.P.P., Y.Z., and P.Z. designed and synthesized CCX721.
Conflict-of-interest disclosure: D.J.D., S.M., J.P.P., L.C.S., Y.W., Y.Z., P.Z., T.J.S., and J.C.J. are employed by and are shareholders of ChemoCentryx Inc, where CCX721 was discovered. The remaining authors declare no competing financial interests.
Correspondence: Daniel J. Dairaghi, ChemoCentryx Inc, 850 Maude Ave, Mountain View, CA 94043; e-mail: ddairaghi@chemocentryx.com.
References
Author notes
D.J.D. and B.O.O. contributed equally to this study.