Abstract
STAT3 plays a crucial role in promoting progression of human cancers, including several types of B-cell lymphoma. However, as a transcription factor lacking its own enzymatic activity, STAT3 remains difficult to target with small-molecule drugs in the clinic. Here we demonstrate that persistent activated STAT3 colocalizes with elevated expression of S1PR1, a G-protein–coupled receptor for sphingosine-1-phosphate (S1P), in the tumor cells of the activated B cell–like subtype of diffuse large B-cell lymphoma patient specimens. Inhibition of S1PR1 expression by shRNA in the lymphoma cells validates that blocking S1PR1 affects expression of STAT3 downstream genes critically involved in tumor cell survival, proliferation, tumor invasion, and/or immunosuppression. Using S1PR1 shRNA, or FTY720, an antagonist of S1P that is in the clinic for other indications, we show that inhibiting S1PR1 expression down-regulates STAT3 activity and causes growth inhibition of the lymphoma tumor cells in vitro and in vivo. Our results suggest that targeting S1P/S1PR1 using a clinically relevant and available drug or other approaches is potentially an effective new therapeutic modality for treating the activated B cell–like subtype of diffuse large B-cell lymphoma, a subset of lymphoma that is less responsive to current available therapies.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is a heterogeneous disease, including subtypes with diverse origins and gene expression profiles.1-6 One of the most aggressive histologies, activated B cell–like DLBCL (ABC-DLBCL) remains a challenge for effective therapy, despite extensive studies of morphology and gene expression patterns.5-10 In vitro, cells of the ABC-DLBCL subtype have gene expression patterns similar to those of activated peripheral blood B cells, such as the NF-κB downstream genes IRF4, CD44, and cyclin D2.11 At the same time, these tumor cells also secrete IL-6 and IL-10 through autocrine/paracrine pathways.12 Consistent with this, the constitutive activation of NF-κB and Janus kinase (JAK) is shown to promote tumorigenicity of malignant B-cell lymphoma, including ABC-DLBCL.13
One promising candidate for ABC-DLBCL targeted therapy is signal transducer and activator of transcription 3 (STAT3). STAT3 is a transcriptional factor that is constitutively activated in many types of cancer, contributing to tumor progression via several mechanisms.14-20 STAT3 is shown to be activated in the ABC-DLBCL subtype.20,21 Approximately one-third of ABC-DLBCL patients have gain-of-function mutations in the adaptor protein MYD88, which leads to JAK and STAT3 activation, as well as IL-6 and IL-10 secretion.13 IL-6 and IL-10 secretion can further increase STAT3 activation levels within tumor cells via an autocrine feedback loop.14 IL-6–activated STAT3 is crucial for survival of several types of cancer cells, including multiple myeloma, a plasmacytic B-cell malignancy.14,22 IL-10 is also important for STAT3-mediated immune suppression in the tumor microenvironment through paracrine mechanisms.15,23 When targeting STAT3 within tumor cells, their proliferation and survival ability decrease concurrently with down-regulation of STAT3 downstream genes that are involved in these processes.14,15
Although STAT3 could be a good candidate for B-cell lymphoma therapy, as a transcriptional factor it is hard to target. Recent discoveries show that STAT3 can also be activated by the sphingosine-1-phosphate receptor 1 (S1PR1), a G-protein–coupled receptor.24 G-protein–coupled receptors are more tractable therapeutic targets than transcriptional factors.25,26 S1PR1 is important for normal B-cell secondary lymphoid organ retention as well as splenic marginal zone localization.27,28 In tumor cells, S1PR1 can maintain persistent activation of STAT3, in part through JAK2 kinase.24 However, it remains unknown whether S1PR1 is crucial for STAT3 activation in ABC-DLBCL. One of the most effective S1PR1 antagonists is FTY720, an analog of the S1PR1 ligand, S1P, with an even higher binding affinity than the natural ligand.29 FTY720 has been in the clinic for certain autoimmune diseases.30,31
Our present study demonstrates that S1PR1 and STAT3 are coactivated in ABC-DLBCL tumor cells. Inhibiting S1PR1 expression using either shRNA, or a small-molecule drug, FTY720, leads to inhibition of STAT3, induction of tumor cell growth arrest and apoptosis. Inhibiting S1PR1 in vivo using both human/mouse xenograft and syngeneic models led to STAT3 inhibition and strong antitumor effects. Our study provides a novel druggable target for certain B-cell lymphoma subtypes that are less responsive to chemotherapy plus rituximab.
Methods
Reagents and cell lines
FTY720 was synthesized by the Synthetic and Biopolymer Chemistry Core at City of Hope. The pLKO.1 nontarget shRNA control, human S1PR1 shRNA lentiviral vectors, and anti–β-actin (AC-15) were purchased from Sigma-Aldrich; anti–Bcl-xL, anti-poly (ADP-ribose) polymerase (H-250), anti-phosphorylated Stat1 (p-Stat1; Tyr701), anti-phosphorylated Stat3 (p-Stat3; Tyr705), anti-phosphorylated Stat5 (p-Stat5; Tyr694), and anti-Stat5 were from Cell Signaling Technology; anti-S1PR1 (A6), anti-Stat1 (E23), and anti-Stat3 (C-20) were from Santa Cruz Biotechnology; and anti-Survivin was from Novus Biologicals. AlexaFluor-488 and AlexaFluor-546 secondary antibodies were purchased from Invitrogen. FITC- and allophycocyanin-conjugated antibodies to annexin V were from BD Biosciences PharMingen. Human ABC-like DLBCL cell lines Ly3 and Ly10 were kind gifts from Dr B. Hilda Ye (Albert Einstein College of Medicine, Bronx, NY) and Dr L. M. Staudt (National Cancer Institute, Bethesda, MD), respectively. Ly3 cells were cultured in IMDM supplemented with 10% FBS. Ly10 cells were cultured in IMDM supplemented with 20% FBS. Murine lymphoma cell line A20 was purchased from ATCC and cultured in RPMI 1640 medium supplemented with 10% FBS.
Lentivirus transduction
The green fluorescent protein (GFP) tagging lentivirus vector eGFP-ffluc_epHIV7 was a gift from Dr Michael Jensen (University of Washington, Seattle, WA). The shRNA part of pLKO.1 nontargeting shRNA control and S1PR1 shRNA lentiviral vectors were subcloned into GFP-tagging lentiviral vectors. The production of lentivirus was performed as previously described.21 Ly3 cells were transduced with shRNA expressing lentivirus, and the infected cells were sorted based on their GFP signals.
Patient specimens and immunohistochemistry and immunofluorescent staining
Paraffin-embedded patient tissue samples were obtained from the archive files of the Pathology Core of City of Hope Comprehensive Cancer Center, with approval from the Institutional Review Board (COH IRB11234). For immunohistochemical staining, paraffin-embedded sections were deparaffinized and hydrated through xylene and graded ethanol series, followed by staining with antibodies against p-Stat3 (Cell Signaling) and S1PR1 (Santa Cruz Biotechnology) and examined under Olympus AX70 automated upright microscope. For immunofluorescent staining, AlexaFluor-488 and AlexaFluor-546 (Invitrogen) were used as secondary antibodies; nuclei were counterstained with Hoechst 33432 (Invitrogen) and examined using Zeiss LSM510 Meta inverted confocal microscope (Carl Zeiss) with 20×/0.5 objectives. The images were captured using Zeiss LSM Image Browser Version 4.2 software and analyzed using Image-Pro plus Version 6.3 (Media Cybernetic Inc). The immunohistochemical staining images were taken on an Aperio ScanScope AT system with 20×/0.75 objectives and analyzed using Aperio ImageScope Version 11.1 software (Aperio Technologies).
In vivo experiments
Murine lymphoma A20 cells (2 × 106/mouse) and ABC-DLBCL Ly3 cells (5 × 106/mouse) transduced with either nontarget shRNA or S1PR1 shRNA were implanted subcutaneously into 6- to 8-week-old male BALB/c (National Cancer Institute) and NOD/SCID IL2Rγ-null mice (The Jackson Laboratory), respectively. A20 tumor-bearing mice were treated by intraperitoneal injection with FTY720 (5 mg/kg body weight) or DMSO vehicle every day, starting when tumors reached 5 to 7 mm in diameter. Mouse care and in vivo experimental procedures were performed under pathogen-free conditions and approved by the Institutional Animal Care and Use Committee of the Beckman Research Institute at City of Hope Medical Center.
Cell proliferation and apoptosis assay
Cells were seeded in triplicates at a density of 5 × 105 cells/mL into 96- or 24-well plates. Cell proliferation assays were performed using the CellTiter 96 AQueous One Solution Cell Proliferation Assay kit (Promega) after treatment at the indicated time. The absorbance at 490 nm was measured using Microplate reader (Bio-Rad). For apoptosis assays, cells were harvested and stained with annexin V–FITC and propidium iodide and assessed for the percentage of double-negative population using an Accuri C6 flow cytometer. For the lentivirus-transduced Ly3 cells, samples were stained with annexin V–allophycocyanin and 4′, 6-diamidino-2-phenylindole. Apoptosis data were analyzed using FlowJo Version 7.6.1 software (TreeStar).
Quantitative real-time PCR and PCR arrays
Total RNA was purified by RNeasy Kit (QIAGEN), and cDNA was synthesized using the iScript cDNA Synthesis Kit (Bio-Rad). Real-time PCR was performed in triplicates using the Chromo4 Real-Time Detector (Bio-Rad). The 18S RNA human housekeeping gene was used as an internal control to normalize the target gene mRNA levels.
Statistical analysis
The correlation between phospho-STAT3 (red) and S1PR1 (green) was quantified based on Manders colocalization coefficients M1 (red to green) and M2 (green to red), using Image-Pro plus Version 6.3 software (Media Cybernetics, Inc). Value ranges from 0 (no colocalization) to 100% (all pixels colocalize). Statistical analysis between 2 groups was calculated as 2-tailed P value using unpaired Student t test.
Results
STAT3 activity correlated with S1PR1 expression in patient ABC-DLBCL tumor cells
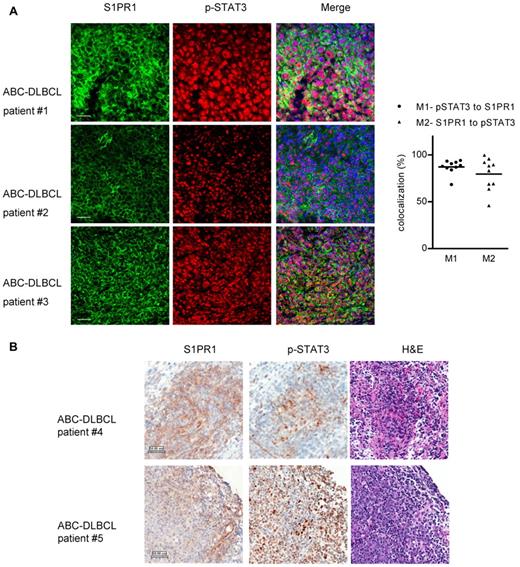
STAT3 is constitutively activated in ABC-DLBCL, but not in GCB-DLBCL lymphoma cells.20 To determine whether S1PR1 expression is crucial for STAT3 activation in ABC-DLBCL tumor cells, we examined S1PR1 and phospho-STAT3 protein levels in a cohort of 10 ABC-DLBCL patient samples. The B-cell lymphoma primary tumor cells showed elevated S1PR1 and STAT3 activity, as determined by immunofluorescent and immunohistochemistry staining (Figure 1A-B). To assess whether S1PR1 was an important contributor for STAT3 activation in primary ABC-DLBCL tumor cells, we quantified S1PR1 expression with phospho-STAT3 (p-STAT3) in the tumor cells, using tissue sections double-stained by S1PR1 and p-STAT3. The expression levels of S1PR1 and p-STAT3 correlated with each other in the same tumor cells within the tumor tissues from these patients, with average colocalization percentages of 87.3 (p-STAT3 to S1PR1) and 79.6 (S1PR1 to p-STAT3), respectively (Figure 1A right). Immunohistochemical staining was performed using separate tissue sections, further supporting the finding that S1PR1 expression was elevated in tumor cells with positive p-STAT3 in ABC-DLBCL (Figure 1B). Consistent with this, ABC-DLBCL cell lines Ly3 and Ly10 also showed elevated STAT3 activity and S1PR1 expression as determined by Western blot analysis (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Coexpression of S1PR1 and phospho-STAT3 in ABC-DLBCL patient tumor cells. (A) Left panel: immunofluorescent staining of S1PR1 (green) and phospho-STAT3 (red) in ABC-DLBCL patient tumor tissue sections. Shown are 3 independent ABC-DLBCL patient tissue sections, representing 10 tested patient samples. Scale bar represents 50 μm. Right panel: quantification of 10 tested patient tissue sections for percentages of overlapping red (p-STAT3) and green (S1PR1) channels, shown as Manders colocalization coefficients M1 (p-STAT3 to S1PR1) and M2 (S1PR1 to p-STAT3), respectively. For each patient tumor section, 10 random fields were chosen to calculate M1 and M2, valued and normalized as mean, which is represented by a dot. One tumor tissue section from each of the 10 patients was analyzed. (B) Immunohistochemistry staining of S1PR1 and phospho-STAT3 of the same region, using consecutive ABC-DLBCL patient tissue sections. Scale bar represents 50 μm.
Coexpression of S1PR1 and phospho-STAT3 in ABC-DLBCL patient tumor cells. (A) Left panel: immunofluorescent staining of S1PR1 (green) and phospho-STAT3 (red) in ABC-DLBCL patient tumor tissue sections. Shown are 3 independent ABC-DLBCL patient tissue sections, representing 10 tested patient samples. Scale bar represents 50 μm. Right panel: quantification of 10 tested patient tissue sections for percentages of overlapping red (p-STAT3) and green (S1PR1) channels, shown as Manders colocalization coefficients M1 (p-STAT3 to S1PR1) and M2 (S1PR1 to p-STAT3), respectively. For each patient tumor section, 10 random fields were chosen to calculate M1 and M2, valued and normalized as mean, which is represented by a dot. One tumor tissue section from each of the 10 patients was analyzed. (B) Immunohistochemistry staining of S1PR1 and phospho-STAT3 of the same region, using consecutive ABC-DLBCL patient tissue sections. Scale bar represents 50 μm.
Specifically targeting S1PR1 using shRNA decreased STAT3-mediated cell proliferation and survival
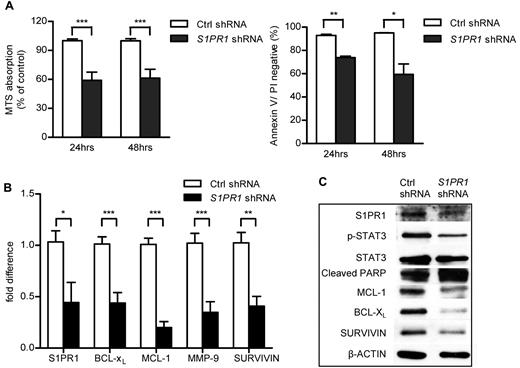
To investigate whether blocking S1PR1 can decrease STAT3 activation and in turn restrain ABC-DLBCL cell proliferation and survival, we transduced Ly3 ABC-DLBCL tumor cells with both control and S1PR1 shRNA-expressing lentiviruses. Specific knockdown of S1PR1 gene expression led to tumor cell growth inhibition and apoptosis (Figure 2A). S1PR1 inhibition reduced STAT3 activity as well as expression of its downstream proliferation/survival-related genes, including proliferation gene MCL-1 and antiapoptotic genes BCL-XL and SURVIVIN, at both mRNA and protein levels (Figure 2B-C). Furthermore, blocking S1PR1/STAT3 signaling not only lowered expression of genes related to proliferation and survival but also those related to extracellular matrix degradation, such as MMP-9 (Figure 2B).
Effects on specific inhibition of S1PR1 in Ly3 ABC-DLBCL cell line. (A) Cell proliferation and apoptosis were assessed 24 or 48 hours after culturing Ly3 tumor cells with or without S1PR1 shRNA knockdown. Data represent 3 independent experiments. ***P < .001. **P < .01. *P < .05. (B) Real-time PCR showing differences in mRNA expression levels of STAT3 downstream genes in control and S1PR1 shRNA lentivirus-transduced Ly3 tumor cells. ***P < .001. **P < .01. *P < .05. Each sample was examined by triplicates. (C) Western blot analysis of protein expression levels of STAT3 downstream genes in both control and S1PR1 shRNA-expressing Ly3 tumor cells. Data represent 3 independent experiments.
Effects on specific inhibition of S1PR1 in Ly3 ABC-DLBCL cell line. (A) Cell proliferation and apoptosis were assessed 24 or 48 hours after culturing Ly3 tumor cells with or without S1PR1 shRNA knockdown. Data represent 3 independent experiments. ***P < .001. **P < .01. *P < .05. (B) Real-time PCR showing differences in mRNA expression levels of STAT3 downstream genes in control and S1PR1 shRNA lentivirus-transduced Ly3 tumor cells. ***P < .001. **P < .01. *P < .05. Each sample was examined by triplicates. (C) Western blot analysis of protein expression levels of STAT3 downstream genes in both control and S1PR1 shRNA-expressing Ly3 tumor cells. Data represent 3 independent experiments.
Inhibiting S1PR1 reduced primary ABC-DLBCL tumor growth and invasion
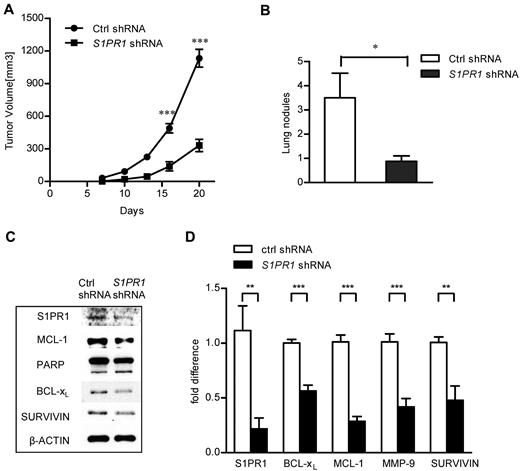
Because blocking S1PR1/STAT3 signaling decreased ABC-DLBCL tumor cell proliferation and apoptosis in culture, we wanted to validate the effects of S1PR1/STAT3 inhibition on tumor progression in vivo. We implanted Ly3 tumor cells, with or without S1PR1 shRNA expression, into NOD/SCID IL2Rγ-null mice, which lack a mouse immune system. Results from this set of experiments showed that specifically blocking S1PR1 expression in human ABC-DLBCL tumor cells decreased primary tumor growth in xenograft models (Figure 3A). Furthermore, one of the biggest obstacles to B-cell lymphoma treatment is invasion/extranodal infiltration of the tumor cells.32 S1PR1 knockdown reduced approximately 75% of the number of lung metastatic nodules in the xenograft tumor model (Figure 3B). Consistent with our in vitro results, blocking S1PR1/STAT3 signaling also down-regulated expression of genes related to proliferation and survival both at mRNA and protein levels in growing tumors (Figure 3C-D).
Specific silencing of S1PR1 in Ly3 ABC-DLBCL tumor cells inhibited tumor growth and invasion in vivo. (A) Ly3 tumor growth was inhibited by specific S1PR1 silencing; N = 8. ***P < .001. Data are representative results from one of 2 independent experiments. (B) Inhibiting S1PR1 by shRNA in ABC-DLBCL tumor cells was accompanied by a reduction in lung invasion in vivo. Lung nodules were numerated on day 20; N = 8. *P < .05. (C) Knocking down S1PR1 reduced expression of STAT3 target genes in vivo. Western blot analysis to detect expression of STAT3 downstream genes at protein level, using whole tumor lysates prepared from tumors grown from control and S1PR1 shRNA expressing Ly3 ABC-DLBCL tumor cells. Eight tumors were pooled to prepare the lysates. (D) Real-time PCR to analyze RNA expression levels of Stat3 downstream genes involved in proliferation, survival, and immune response in tumors grown from control and S1PR1 shRNA-transduced tumor cells. Eight tumors were pooled to prepare RNA, and real-time PCR was performed as triplicates for each sample. ***P < .001; **P < .01.
Specific silencing of S1PR1 in Ly3 ABC-DLBCL tumor cells inhibited tumor growth and invasion in vivo. (A) Ly3 tumor growth was inhibited by specific S1PR1 silencing; N = 8. ***P < .001. Data are representative results from one of 2 independent experiments. (B) Inhibiting S1PR1 by shRNA in ABC-DLBCL tumor cells was accompanied by a reduction in lung invasion in vivo. Lung nodules were numerated on day 20; N = 8. *P < .05. (C) Knocking down S1PR1 reduced expression of STAT3 target genes in vivo. Western blot analysis to detect expression of STAT3 downstream genes at protein level, using whole tumor lysates prepared from tumors grown from control and S1PR1 shRNA expressing Ly3 ABC-DLBCL tumor cells. Eight tumors were pooled to prepare the lysates. (D) Real-time PCR to analyze RNA expression levels of Stat3 downstream genes involved in proliferation, survival, and immune response in tumors grown from control and S1PR1 shRNA-transduced tumor cells. Eight tumors were pooled to prepare RNA, and real-time PCR was performed as triplicates for each sample. ***P < .001; **P < .01.
Blocking S1PR1/STAT3 signaling using S1PR1 inhibitor FTY720 induced ABC-DLBCL tumor cell growth inhibition and apoptosis
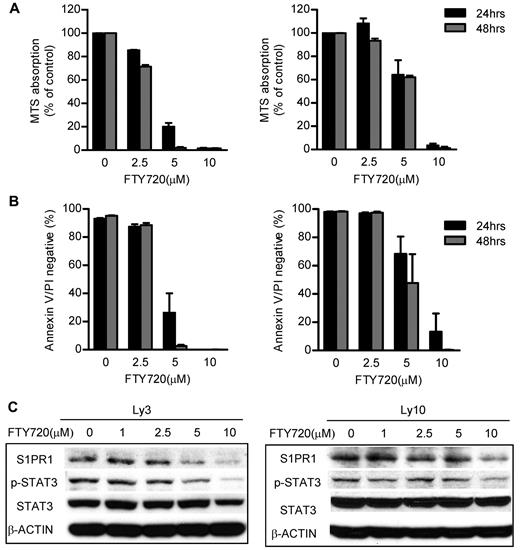
Because directly blocking S1PR1 by shRNA can decrease ABC-DBCL tumor growth, next we assessed whether using the S1PR1 antagonist FTY720 had antitumor therapeutic effects. Treatment of FTY720 reduced tumor cell growth in 24 hours as determined by MTS assay of metabolic rate. The growth inhibition reached more than 95%, after treatment of Ly3 ABC-DLBCL tumor cells with 5μM FTY720 for 48 hours (Figure 4A left panel). Ly10 ABC-DLBCL tumor cells required relatively higher concentrations (10μM) of FTY720 treatment to show profound growth inhibition (Figure 4A right panel). FTY720 treatment at 5-10μM range also reduced cell viability, as determined by annexin V and propidium iodide double-negative staining (Figure 4B). Because IL-6 and IL-10 are important STAT3 activators and are secreted by ABC-DLBCL tumor cells, we further tested whether blocking S1PR1 by FTY720 could inhibit production of these cytokines. Results showed that IL-6 was reduced by FTY720 in Ly3 tumor cells (Ly10 tumor cells do not produce detectable IL-6). IL-10 secretion was also reduced by FTY720 treatment in both Ly3 and Ly10 tumor cells (supplemental Figure 2).
S1PR1 antagonist FTY720 induced apoptosis and growth inhibition of ABC-DLBCL tumor cells through abrogating S1PR1/STAT3 signaling. (A) FTY720 treatment inhibited ABC-DLBCL tumor cell proliferation. ABC-DLBCL cell lines, Ly3 and Ly10, were treated with FTY720 at different concentrations as indicated for 24 or 48 hours. The relative cell numbers of different treatments were determined by MTS assay. (B) Treating ABC-DLBCL tumor cells with FTY720 induced apoptosis. The percentage of viable cells was determined by flow cytometry to detect annexin V and propidium iodide (PI) double-negative cells. (C) FTY720 treatment inhibited STAT3 activity in ABC-DLBCL tumor cells. The levels of S1PR1 and phospho-STAT3 in Ly3 and Ly10 ABC-DLBCL cells after FTY720 treatment (6 hours) were determined by Western blot analysis. All data are representative of results from 3 independent experiments.
S1PR1 antagonist FTY720 induced apoptosis and growth inhibition of ABC-DLBCL tumor cells through abrogating S1PR1/STAT3 signaling. (A) FTY720 treatment inhibited ABC-DLBCL tumor cell proliferation. ABC-DLBCL cell lines, Ly3 and Ly10, were treated with FTY720 at different concentrations as indicated for 24 or 48 hours. The relative cell numbers of different treatments were determined by MTS assay. (B) Treating ABC-DLBCL tumor cells with FTY720 induced apoptosis. The percentage of viable cells was determined by flow cytometry to detect annexin V and propidium iodide (PI) double-negative cells. (C) FTY720 treatment inhibited STAT3 activity in ABC-DLBCL tumor cells. The levels of S1PR1 and phospho-STAT3 in Ly3 and Ly10 ABC-DLBCL cells after FTY720 treatment (6 hours) were determined by Western blot analysis. All data are representative of results from 3 independent experiments.
We next determined whether blocking S1PR1 using FTY720 could decrease STAT3 activity. We treated Ly3 and Ly10 tumor cells with FTY720 at the same concentrations used for proliferation and apoptosis assays to determine S1PR1 and phospho-STAT3 expression levels. FTY720 treatment lowered S1PR1 levels 6 hours after treatment at a minimum concentration of 5μM. Phospho-STAT3 was also decreased, accompanied by S1PR1 down-regulation (Figure 4C). Adding a JAK inhibitor, AZD1480, at 0.5μM to FTY720 treatment, had some additive effect on STAT3 inhibition (supplemental Figure 3). Decreasing of p-STAT3 by FTY720 also reduced the expression of STAT3 downstream genes critical for proliferation and survival, including MCL-1 and BCL-XL (supplemental Figure 4A). To test the specificity and potential effects of FTY720 on the activity of other STAT family proteins, we assessed pSTAT1 and pSTAT5 levels in Ly3 and Ly10 tumor cells, before and after FTY720 treatment. However, the levels of pSTAT1 and pSTAT5 were not readily detectable in these tumor cells, as determined by Western blotting (supplemental Figure 4B).
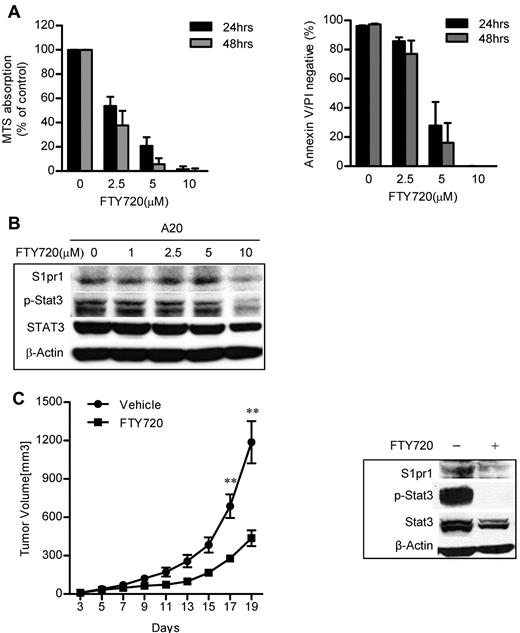
FTY720 inhibited Stat3 activity and tumor growth in murine A20 lymphoma in vivo
Because the tumor microenvironment, especially the immunologic microenvironment, plays an important role during tumor progression and therapy, we next used murine A20 B-cell lymphoma cells, which were derived from a BALB/c mouse, to validate whether S1PR1 could be a good therapeutic target in both tumor cells as well as tumor stromal immune cells. Consistent with the data from human ABC-DLBCL cell lines, treatment of the mouse B-cell lymphoma A20 cell line with FTY70 also reduced cell proliferation and survival (Figure 5A), abrogated S1pr1 expression, and Stat3 activity (Figure 5B). To determine whether FTY720 could inhibit tumor growth through targeting S1PR1/STAT3 signaling, A20 B-cell lymphoma cells were implanted into syngeneic BALB/c mice and treated with FTY720. Subcutaneous A20 tumors in mice treated by intraperitoneal injection with FTY720 daily at 5 mg/kg led to a reduction of greater than 50% of tumor growth in vivo (Figure 5C left panel). Consistent with in vitro treatment results, FTY720 inhibited Stat3 activity in tumors in vivo, which was coupled with a reduction in S1pr1 expression (Figure 5C right panel). Notably, total Stat3 levels were also decreased in the whole tumor after FTY720 treatment (Figure 5C right panel), which is probably the result of the ability of phospho-Stat3 to autoregulate Stat3 expression.
Targeting S1pr1/Stat3 signaling inhibited murine B-cell lymphoma tumor growth in a syngeneic mouse model. (A) Inhibition of S1pr1 induced apoptosis and growth inhibition of the murine B-cell lymphoma A20 cell line in vitro. A20 B-cell lymphoma cells were treated with FTY720 at indicated concentrations. Tumor cell proliferation and apoptosis were examined 24 or 48 hours after treatment. The results represent 3 independent experiments. (B) S1pr1 and phospho-Stat3 levels in A20 tumor cells were determined by Western blot analysis after treating with FTY720 for 6 hours. (C) A20 tumor growth and Stat3 activity were decreased by FTY720 treatment in vivo. Left panel: tumor growth curve showing that FTY720 inhibited A20 tumor growth in vivo; N = 5. **P < .01. Data are representative of 2 independent experiments. Right panel: Western blotting analysis of S1pr1 and phospho-Stat3 protein levels in A20 tumors after FTY720 treatment in vivo. Five tumors were pooled to make protein lysates. Data are representative of 2 independent experiments.
Targeting S1pr1/Stat3 signaling inhibited murine B-cell lymphoma tumor growth in a syngeneic mouse model. (A) Inhibition of S1pr1 induced apoptosis and growth inhibition of the murine B-cell lymphoma A20 cell line in vitro. A20 B-cell lymphoma cells were treated with FTY720 at indicated concentrations. Tumor cell proliferation and apoptosis were examined 24 or 48 hours after treatment. The results represent 3 independent experiments. (B) S1pr1 and phospho-Stat3 levels in A20 tumor cells were determined by Western blot analysis after treating with FTY720 for 6 hours. (C) A20 tumor growth and Stat3 activity were decreased by FTY720 treatment in vivo. Left panel: tumor growth curve showing that FTY720 inhibited A20 tumor growth in vivo; N = 5. **P < .01. Data are representative of 2 independent experiments. Right panel: Western blotting analysis of S1pr1 and phospho-Stat3 protein levels in A20 tumors after FTY720 treatment in vivo. Five tumors were pooled to make protein lysates. Data are representative of 2 independent experiments.
Discussion
Although STAT3 is a promising target for cancer therapy, effectively inhibiting the activity of a transcriptional factor remains a challenge because of their intracellular localization and lack of enzymatic activity. Recently, a G-protein–coupled receptor, S1PR1, is discovered to be important for persistent activation of STAT3 in certain mouse tumors and human tumor cells.24 S1PR1 is shown to physically interact with JAK2, leading to increased STAT3 phosphorylation. Activated STAT3 can in turn up-regulate S1PR1 expression at the transcriptional level, thereby forming a feed-forward loop.24 STAT3 activation is also contributed by IL-6 and other factors, leading to further S1PR1 (over)-expression. Compared with STAT3, S1PR1, as a GRCR, has a more advantageous cellular localization as well as the capacity for enzymatic targeting. However, the importance of S1PR1 in cancer biology is just beginning to be appreciated, and its potential importance as a target for human cancer therapy remains to be further assessed. Our study shows that STAT3 activation and S1PR1 expression colocalize in patient ABC-DLBCL tumor cells.
S1PR1 is crucial for lymphocyte secondary lymphoid organ retention for both T lymphocytes and B lymphocytes.28,33 S1PR1 is also highly expressed in endothelial cells and pericytes, and the expression of S1PR1 within these cells is important for tumor angiogenesis and metastasis.34,35 On the other hand, blocking S1PR1 can retain lymphoma cells in lymphoid organs because of an egress function defect.29,36 This could potentially limit the lymphoma cells' invasive potential. Our results demonstrate the effectiveness of the S1PR1 antagonist FTY720, which has already been used clinically for treatment of multiple sclerosis.30,31 This drug is also shown to be effective in many types of cancer in animal models.37-39 We show that this clinically available agent can inhibit ABC-DLBCL cell proliferation and survival in vitro through decreasing STAT3 activity.
Further preclinical studies on the feasibility of using FTY720 to treat B-cell lymphoma patients are necessary. It will be especially desirable to study the effect of blocking S1PR1/STAT3 signaling in both tumor cells and in the tumor immunologic environment using humanized mouse models with human B-cell lymphoma cells implanted. In addition to FTY720, a promising drug to target this pathway, is an antibody against S1P ligand, which has been shown to effectively inhibit primary tumor growth in various mouse models and has recently entered clinical trials for other indications.35,40 Another possibility is the use of a novel in vivo siRNA delivery technology, CpG oligonucelotide-conjugated siRNA, to specifically target Toll-like receptor 9-positive cells.41,42 Toll-like receptor 9-positive cells include normal macrophages and B cells and various types of malignant myeloid and B cells, including ABC-DLBCL. Using CpG-S1PR1 siRNA, one could target not only S1PR1/STAT3 signaling in tumor cells but concurrently enhance the antitumor immune response by activating tumor-associated dendritic cells, macrophages, and B cells. Taken together, our results suggest that S1PR1 can be a viable target, via inhibiting STAT3 activity, for treating ABC-DLCL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank staff members at Pathology Core, Light Microscopy Core, Animal Facility Core, Flow Cytometry Core at Beckman Research Institute City of Hope Comprehensive Cancer Center for their excellent technical support as well as Sandra Thomas at Department of Hematology at City of Hope for editing the paper.
This work was supported by Tim Nesviq Fund at City of Hope Comprehensive Cancer Center, the National Institutes of Health (grants P50CA107399 and R01CA146092), the V Foundation Translational Research Grant, and HEADstrong Foundation, in memory of Nicholas E. Colleluori.
National Institutes of Health
Authorship
Contribution: H.Y. conceptualized the project and helped to design the experiments and manuscript writing; Y.L. and J.D. performed the majority of the experiments, analyzed the data, and prepared the manuscript; L.W. contributed to the immunofluorescent staining; H.L. also helped the design of experiments and data interpretation; B.A. contributed to imaging scanning for the statistical analysis of immunofluorescent staining data; A.S. contributed to the study of human ABC-DLBCL cell lines; C.K. performed the lentivirus transduction experiments; and L.M.W. and S.F. assessed patient specimens and/or provided clinical relevance.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hua Yu, Department of Cancer Immunotherapeutics and Tumor Immunology, Beckman Research Institute, 1500 E Duarte Rd, Duarte, CA 91010; e-mail: hyu@coh.org.
References
Author notes
Y.L. and J.D. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal