Key Points
C3 and MDSC development
Abstract
Myeloid-derived suppressor cells (MDSCs) play an important role in the regulation of the immune response. MDSC expansion occurs in many circumstances, including cancer, inflammation, stresses, and transplant tolerance. Liver transplants in mice are spontaneously accepted, but hepatocyte transplants are acutely rejected, suggesting the immunoregulatory activities of liver nonparenchymal cells. We have reported that hepatic stellate cells (HpSCs), the stromal cells in the liver, are immensely immunosuppressive and can effectively protect islet transplants via induction of MDSCs. The present study shows that the addition of HpSCs into dendritic cell (DC) culture promoted development of MDSCs, instead of DCs, which was highly dependent on complement component 3 (C3) from HpSCs. The C3−/− HpSCs lost their ability to induce MDSCs and, consequently, failed to protect the cotransplanted islet allografts. HpSCs produced complement activation factor B and factor D which then enhanced C3 cleavage to activation products iC3b and C3d. Addition of exogenous iC3b, but not C3d, into the DC culture led to the differentiation of MDSCs with potent immune-inhibitory function. These findings provide novel mechanistic insights into the differentiation of myeloid cells mediated by local tissue cells, and may assist in the development of MDSC-based therapy in clinical settings.
Introduction
Myeloid-derived suppressor cells (MDSCs) were initially identified in cancer for their ability to negatively regulate the immune system, contributing to tumor persistence and metastasis.1 MDSCs are not a defined subset of myeloid cells but rather a heterogeneous population of progenitor cells consisting of macrophages, granulocytes, dendritic cells (DCs), and immature myeloid cells.2 The variation of cell types within the MDSC population and lack of reliable phenotypic markers makes identification difficult. However, MDSCs share many unifying features, including high expression of the immunosuppressive molecules arginase 1 and inducible nitric oxide synthase (iNOS), and, more importantly, their immunosuppressive function.3 Evidence for the immunoregulatory mechanisms of MDSCs has been demonstrated in acute and chronic infection, sepsis, trauma, autoimmune diseases, and transplantation models, in which an increased frequency of MDSCs was observed in relevant tissues, suggesting that the expansion of MDSCs correlates with the modulation of the inflammatory response in many conditions.2-5
The potent immunosuppressive activity of MDSCs makes them attractive candidates for use in cell therapy of autoimmune diseases. Thus far, efforts to develop MDSC-based therapeutic strategies have been hampered by the lack of reliable methods to generate sufficient numbers of immunosuppressive MDSCs in vitro.6 It has been noted that liver allografts are spontaneously accepted across fully mismatched major histocompatibility complex barriers in mice, while hepatocyte transplants in the same strain combinations are acutely rejected,7 ,8 suggesting that liver nonparenchymal cells are contributing to the immunoregulatory properties of the liver. Examination of various liver nonparenchymal cells showed that the hepatic stellate cell (HpSC), a type of stromal cell in the liver well known for its role in storing vitamin A and participating in fibrosis following liver injury, is immensely immunosuppressive.9 Cotransplantation of HpSCs with islet allografts effectively protected the islet allografts from rejection without the requirement of immunosuppression via inhibition of CD8+ T-cell responses, enhancement of regulatory T (Treg) cells,10 ,11 and induction of MDSCs.12 HpSCs have a limited capacity to directly induce Treg cells13 but are potent inducers of MDSCs.12 The direct link between HpSCs and MDSCs was demonstrated by addition of small amounts of HpSCs into DC (bone marrow [BM] derived) culture, leading to generation of MDSCs instead of DCs. These newly generated MDSCs showed potent immunosuppressive activity, and protected islet allografts as effectively as HpSCs, although the number of MDSCs required to generate the same suppressive effect as HpSCs was 10 times greater.12
To explore the molecular basis through which HpSCs promote MDSC development, HpSCs were cultured with BM cells in a transwell system. Despite the lack of direct cell contact, HpSCs were still capable of inducing MDSCs through the production of soluble factors. This finding was reproduced in conditions where HpSC culture supernatant was added to BM cells and shown to facilitate MDSC generation. However, BM cells cultured in the presence of heat-treated supernatant limited the development of MDSCs, indicating that HpSC-soluble factors are composed of protein components.12 Upon activation, HpSCs produce vascular endothelial growth factor (VEGF), granulocyte macrophage colony-stimulating factor (GM-CSF), and granulocyte colony-stimulating factor (G-CSF). These factors have previously been shown to promote MDSCs in the cancer environment.2 ,10 However, these factors do not appear to be critical in the induction of MDSCs by HpSCs.12 To identify the responsible factor(s), HpSCs were cultured in serum-free medium to minimize the influence of serum proteins. Culture in serum-free medium did not compromise the ability of HpSCs to promote MDSCs.12 Analysis of the size-fractioned HpSC culture supernatant revealed that the 100- to 250-kDa portion had the most bioactivity and had a greater influence on MDSC induction. Electrophoresis analysis (sodium dodecyl sulfate–polyacrylamide gel electrophoresis) showed the presence of unique bands in the HpSC culture supernatant that were absent in the control. Peptide sequence analysis of the bands identified 2 groups of molecules: (1) extracellular matrices, which were expected, and (2) complement, including complement component 3 (C3), which was beyond expectation since C3 is mainly produced by hepatocytes. Protein analysis of the HpSC culture supernatant through western blot analysis confirmed the bands of C3 subunits C3α and β.12 In the present study, we show that HpSCs from mice deficient in C3 (C3−/−) fail to protect islet allografts and cannot induce MDSCs. HpSCs equip the complement activation factors B and D. We further demonstrate that the induction of MDSCs by C3 is likely mediated by iC3b, an activation/degradation product of C3 cleavage, since addition of exogenous iC3b into the DC culture promoted generation of MDSCs.
Materials and methods
Animals
Male C57BL/6J (B6), BALB/c mice were purchased from The Jackson Laboratory (Bar Harbor, ME). C3 knockout mice were kindly provided by Dr Edward Medof (Case Western Reserve University, Cleveland, OH), and were originally purchased from The Jackson Laboratory. All animals were maintained following National Institutes of Health guidelines, and animal studies have been reviewed and approved by an institutional review committee.
Preparation of HpSCs
HpSCs were isolated from mouse livers as previously described.9 ,14 Livers were perfused with phosphate-buffered saline and type IV collagenase, and soaked in collagenase for further digestion. The HpSCs were isolated and enriched by Percoll gradient centrifugation and cultured (105/mL) in cell-culture flasks (25 cm2 surface area) (Nunclon, Roskilde, Denmark) with RPMI 1640 (Mediatech Inc, Herndon, VA) supplemented with 20% volume to volume ratio heat-inactivated fetal calf serum in 5% CO2/95% air at 37°C for 7 to 10 days. Cell viability was >90% as determined by trypan blue exclusion. The purity of HpSCs was >95% determined by desmin immunostaining and by the typical light microscopic appearance of lipid droplets. Contamination of hepatocytes was ruled out by morphologic examination and lack of detectable albumin in the culture supernatant (enzyme-linked immunosorbent assay). Flow analysis confirmed the absence of CD45+ cells which ruled out contamination of macrophages.
Culture of DCs
As previously described,15 ,16 2 × 106 BM cells per well from tibias and femurs of B6 mice were cultured in RPMI 1640 medium containing 10% fetal calf serum in the presence of mouse recombinant GM-CSF (8 ng/mL; Schering-Plough, Kenilworth, NJ) for 5 days (hereafter referred to as DCs). To test the impact of C3 produced by HpSCs, wild-type (WT) or C3−/− HpSCs were added at the beginning of the DC culture at a HpSC:BM cell ratio of 1:80. The floating cells were harvested, washed, and resuspended in RPMI 1640 medium. To study the function of these generated myeloid cells, CD11b+ cells (myeloid cells) were then purified by positive selection with magnetic-activated cell sorting microbeads (Miltenyi Biotec, Auburn, CA).
Flow cytometric analysis
The monoclonal antibodies (mAbs) against CD4, CD8, CD11b, CD11c, CD25, CD40, CD86, F4/80, B220, Ly6C, Gr-1, H2Kb, I-Ab, and interferon-γ (IFN-γ) were purchased from BD PharMingen (San Diego, CA), against B7-H1 and Foxp3 from eBioscience (San Diego, CA), against C3 and C3b from Quidel (San Diego, CA). Intracellular staining protocols were followed for staining of IFN-γ or Foxp3. For carboxyfluorescein diacetate succinimidyl ester (CFSE) labeling, T cells (107/mL) were incubated with 0.5μM CFSE (Molecular Probes, Eugene, OR) for 10 minutes at room temperature. Flow analyses were performed with a BD FACSCalibur flow cytometer (BD Biosciences, San Jose, CA).
Immunohistochemistry
CD4 and CD8 cells in cryostat sections were identified by fluorescent staining using specific mAbs (BD PharMingen). Desmin and C3 were stained using rabbit anti-mouse desmin (Millipore, Billerica, MA) and goat anti-mouse C3 Ab (MP Biomedicals, Solon, OH) following permeabilization with 0.05% saponin buffer using the Vectastain Elite ABC kit (Vector Laboratories Inc, Burlingame, CA) as immunoperoxidase. The slides were developed by 3-amino-9-ethylcarbazole chromogen/substrate and counterstained with hematoxylin. The isotype and species matched irrelevant Abs served as controls. For quantification, the cells were counted under a microscope, and a total of 10 high-power fields (hpf’s) were randomly selected.
Pancreatic islet transplantation
As previously described,10-12 300 pancreatic islets (BALB/c) alone or mixed with 2.5 × 105 WT or C3−/− HpSCs (B6) (the optimal dose as previously shown10 ) were transplanted under the renal capsule of streptozotocin-induced diabetic recipients (B6). No immunosuppressive drugs were administered throughout the experiments. Transplantation was considered successful when blood glucose became normal (<150 mg/dL) for at least 4 days. The first day of 2 consecutive readings of blood glucose >350 mg/dL was defined as graft failure. In all animals with euglycemia for >60 days, the kidneys bearing islet grafts were removed, resulting in a prompt return to hyperglycemia, indicating the function of the islet grafts. For mechanistic studies, the animals were sacrificed on postoperation day (POD) 7.
Mixed leukocyte reaction
One-way mixed leukocyte reaction (MLR) culture was performed in triplicate in a 96-well, round-bottom microculture plate (Corning, Corning, NY). Nylon wool-eluted splenic T cells (2 × 105 per well) from B6 mice were used as responders. T-cell proliferation was elicited either by anti-CD3 mAb (5 μg/mL) or by graded doses of γ-irradiated (20 Gy; x-ray source) indicated stimulator cells. Cultures were maintained in RPMI 1640 complete medium for 3 to 5 days in 5% CO2. T-cell proliferative response was determined by either thymidine uptake or CFSE dilution assay analyzed by flow cytometry. IFN-γ production was determined by intracellular staining using specific mAb. To examine the suppressive effect of generated cells on T-cell proliferation, the irradiated cells (or control cells) were added as regulators into the MLR culture at the indicated ratio of stimulators:regulators.
Generation of cytotoxic T-lymphocyte assay
BALB/c spleen T cells (2 × 105 per well in 100 μL) were cultured with γ-irradiated (20 Gy) CD11b+ (myeloid) cells isolated from islet grafts at a ratio of 5:1 for 5 to 6 days then used as effectors. EL4 (H2b), R1.1 (H2k), or P815 (H2d) lymphoma cells (4 × 106, all from American Type Culture Collection, Rockville, MD) were labeled with 5μM fluorescent dye 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein (BCECF) (Invitrogen, Carlsbad, CA) as described in the manufacturer’s instruction, used as donor-specific, syngeneic, or third-party targets, and plated in 96-well round-bottom culture plates (5 × 103 cells/ well). Serial twofold dilutions of effector cells were added. The BCECF released in the supernatant resulted from lysis of the target cells and was measured by a fluorescence microtiter plate reader (Molecular Devices, Sunnyvale, CA), expressed as the mean ± SD of percentage-specific BCECF release in triplicate cultures.
PCR assay
Total RNA was extracted with TRIzol Reagent. Complementary DNA (cDNA) was synthesized with SuperScript II reverse transcriptase (Invitrogen). Quantitative polymerase chain reaction (qPCR) primers were arginase-1: forward CACGGCAGTGGCTTTAACCT, reverse TGGCGCATTCACAGTCACTT; iNOS: forward TGGCCACCTTGTTCAGCTACG, reverse GCCAAGGCCAAACACAGCATAC. The messenger RNAs (mRNAs) were measured using the 7500 Fast PCR system (Applied Biosystems, Foster City, CA) in duplicate, and were normalized to 18S mRNA. For semiquantitative PCR, the primers for factor B were forward TCTGGTGGACTCCGTGAACATCAA, reverse TGAGCTTGACTAGGGCCACATCAT; the primers for factor D were forward TGATGTGCAGAGTGTAGTGCCTCA, reverse ACGTAACCACACCTTCGACTGCAT. PCR was performed by using GeneAmp PCR system 2700 (Applied Biosystems).
Alternative pathway (factor B and D) compensation assay
Zymosan A is prepared from the yeast cell wall and consists of insoluble protein-carbohydrate complexes. Zymosan can activate complement through the alternative pathway, which is dependent on factors B and factor D. After C3 activation, the activation products C3b/iC3b/C3d (not C3) can covalently bind on the zymosan surface, which can be quantitatively assessed by flow cytometry following anti-C3 Ab staining. Because C3 is unable to bind to zymosan, no binding can be detected when zymosan is incubated in serum from factor B−/− or factor D−/− mice.17 ,18 This assay was used to determine whether HpSC produced functional factor B and factor D proteins by addition of HpSC culture supernatant into factor B−/− or factor D−/− serum. Zymosan A (0.125 mg/mL; Sigma-Aldrich, St. Louis, MO) was incubated with serum (with or without compensated with HpSC conditioning medium) for 15 minutes at 37°C before flow analysis.9
Statistical analysis
Graft survival between groups of animals that received transplants was compared using the log-rank test. The parametric data were analyzed by the Student t test (2-tailed). Values of P < .05 were considered statistically significant.
Results
C3 produced by HpSCs participates in induction of MDSCs in vitro
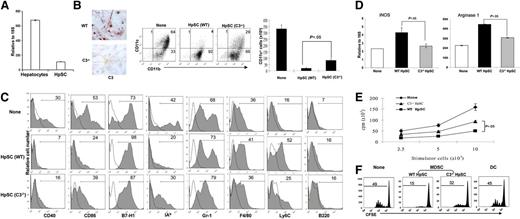
qPCR analysis showed that C3 mRNA expression in HpSCs was approximately one-seventh of that in hepatocytes (Figure 1A), slightly higher than that in macrophages, which were previously reported to produce C3 ∼15 times less than heptocytes.19 To definitively determine the role of C3 produced by HpSCs in induction of MDSCs, HpSCs isolated from C3−/− mice were used, which were confirmed to be C3-negative by immunochemical staining (Figure 1B left panels). The WT or C3−/− HpSCs were added into the DC culture in serum-free medium for 5 days. The floating cells were harvested. Addition of either WT or C3−/− HpSCs did not affect the total number of developing cells, as well as cell survival (determined by terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling staining, data not shown). However, addition of WT HpSCs resulted in a significant reduction in DC development (CD11c+ cells declined from ∼65% in no HpSC control to ∼5%), but deviation toward CD11b+CD11c− cells, which we have previously shown to mainly contain MDSCs.12 Addition of C3−/− HpSCs markedly restored the progenitors’ ability to generate DCs (the incidence of CD11c+ cells increased to ∼30%), and inhibited the differentiation of MDSCs (CD11b+CD1c−) (Figure 1B left panels). These trends were confirmed by comparison of the absolute number of CD11c+ cells (Figure 1B right panel). Figure 1C shows flow analysis for expression of the key surface molecules gated on the CD11b+ population. Compared with no HpSC controls, the myeloid (CD11b+) cells generated in the WT HpSC group were phenotypically immature (expressing low costimulatory molecules CD40 and CD86), with enhanced expression of Ly6C, suggesting that HpSCs induce the monocytic subset of MDSCs.20 Furthermore, these cells expressed intermediate levels of F4/80 (macrophage) and low levels of B220 (B cells and/or plasmacytoid DCs), reflecting their heterogeneous nature. The myeloid cells generated in the presence of C3−/− HpSCs exhibited a more mature phenotype and expressed less Ly6C compared with the WT HpSC group, suggesting a reduced capacity for inducing MDSCs. Addition of WT or C3−/− HpSCs did not have a significant impact on expression of Gr-1; therefore, Gr-1 is unlikely a reliable marker for MDSCs in this experimental setting. This is consistent with our previous observations.12 ,21
Impact of C3 produced by HpSCs on induction of MDSCs in vitro. (A) mRNA expression of C3 in hepatocytes and HpSCs from normal B6 mice were determined by qPCR assay. (B) Impact of C3 produced by HpSCs on myeloid cell development. HpSCs prepared from C3−/− or WT B6 mice were stained with anti-C3 Ab (red) and examined under a microscope (left panels). HpSCs from C3−/− or WT mice were added at the beginning of B6 BM cell culture at a ratio of 1:80 in the presence of GM-CSF for 5 days. Culture without HpSCs served as a control. The floating cells were harvested, stained for CD11b, CD11c, and analyzed by flow cytometry (middle panels). The number represents the percentage of the positive cells in the whole-cell population. The absolute number of CD11c+ cells per well were calculated based on flow analysis (right panel). (C) Expression of key surface molecules. The cells were analyzed by gating on CD11b+ cells and expressed as histograms. The clear areas are isotype controls. The number is a percentage of the positive cells in the CD11b+ cells. (D) Expression of iNOS and arginase 1. CD11b+ cells were purified (magnetic beads), examined for mRNA expression of iNOS and arginase 1 by qPCR, and expressed as a mean ± 1 SD (n = 3). (E) Allostimulatory activity. BALB/c spleen T cells (2 × 105 per well) were cultured with a graded number of irradiated CD11b+ cells in triplicate for 3 days. The proliferative response was determined by 3H-thymidine incorporation and expressed as mean cpm ± 1 SD. (F) CD11b+ cells from the C3−/− HpSC group largely lose the ability to inhibit the T-cell proliferative response. In an MLR culture, CFSE-labeled B6 spleen T cells were cultured with irradiated BALB/c DCs at a ratio of 20:1 for 3 days. CD11b+ cells purified from the DC culture in the presence of WT or C3−/− HpSCs (referred as MDSCs) were added at the beginning into the MLR culture at a DC:MDSC ratio of 1:1. Addition of DCs, instead of MDSCs, served as control. The proliferative response was determined by CFSE dilution gated in the CD3+ population. The data are representative of 3 separate experiments.
Impact of C3 produced by HpSCs on induction of MDSCs in vitro. (A) mRNA expression of C3 in hepatocytes and HpSCs from normal B6 mice were determined by qPCR assay. (B) Impact of C3 produced by HpSCs on myeloid cell development. HpSCs prepared from C3−/− or WT B6 mice were stained with anti-C3 Ab (red) and examined under a microscope (left panels). HpSCs from C3−/− or WT mice were added at the beginning of B6 BM cell culture at a ratio of 1:80 in the presence of GM-CSF for 5 days. Culture without HpSCs served as a control. The floating cells were harvested, stained for CD11b, CD11c, and analyzed by flow cytometry (middle panels). The number represents the percentage of the positive cells in the whole-cell population. The absolute number of CD11c+ cells per well were calculated based on flow analysis (right panel). (C) Expression of key surface molecules. The cells were analyzed by gating on CD11b+ cells and expressed as histograms. The clear areas are isotype controls. The number is a percentage of the positive cells in the CD11b+ cells. (D) Expression of iNOS and arginase 1. CD11b+ cells were purified (magnetic beads), examined for mRNA expression of iNOS and arginase 1 by qPCR, and expressed as a mean ± 1 SD (n = 3). (E) Allostimulatory activity. BALB/c spleen T cells (2 × 105 per well) were cultured with a graded number of irradiated CD11b+ cells in triplicate for 3 days. The proliferative response was determined by 3H-thymidine incorporation and expressed as mean cpm ± 1 SD. (F) CD11b+ cells from the C3−/− HpSC group largely lose the ability to inhibit the T-cell proliferative response. In an MLR culture, CFSE-labeled B6 spleen T cells were cultured with irradiated BALB/c DCs at a ratio of 20:1 for 3 days. CD11b+ cells purified from the DC culture in the presence of WT or C3−/− HpSCs (referred as MDSCs) were added at the beginning into the MLR culture at a DC:MDSC ratio of 1:1. Addition of DCs, instead of MDSCs, served as control. The proliferative response was determined by CFSE dilution gated in the CD3+ population. The data are representative of 3 separate experiments.
To compare the function of myeloid cells generated under the influence of WT or C3−/− HpSCs, CD11b+ (myeloid) cells were purified (positive selection using magnetic beads) and examined for expression of iNOS and arginase 1 by qPCR. CD11b+ cells from the C3−/− HpSC group demonstrated a significant reduction in expression of iNOS and arginase 1 compared with CD11b+ from WT HpSC controls (Figure 1D). The immunostimulatory activity was assessed in a 1-way MLR. CD11b+ cells purified from the culture with C3−/− HpSCs elicited a higher proliferative response in allogeneic T cells compared with myeloid cells from the WT HpSC group (Figure 1E). Addition of CD11b+ cells from the WT HpSC group into a 1-way MLR culture markedly inhibited T-cell proliferative response as shown by CFSE dilution assay, while the inhibition was attenuated when the added cells were replaced by the CD11b+ cells from the C3−/− HpSC group. T-cell inhibition was not due to overcrowding of cells in the culture because addition of an equivalent number of DCs showed normal T-cell proliferation (Figure 1F). These in vitro data indicate that C3 produced by HpSCs plays an important role not only in inhibiting development of DCs but also in promoting generation of MDSCs.
To examine the dose-dependent effect of HpSC-produced C3 on differentiation of MDSCs, we added mixed HpSCs from C3−/− and WT mice with graded ratios. The data presented in supplemental Figure 1A (available on the Blood website) demonstrate a dose-related effect of HpSC-produced C3. Addition of the HpSCs at a ratio of WT vs C3−/− HpSCs of 1:3 (75% reduction in C3) markedly increased CD11c+ cell expansion (comparable with the C3−/− HpSC-only group), but caused reduction in CD11c− cell induction, which correlated with an increase in stimulating T-cell proliferation (supplemental Figure 1).
C3 is required for HpSCs to exert immune regulatory activity in vivo
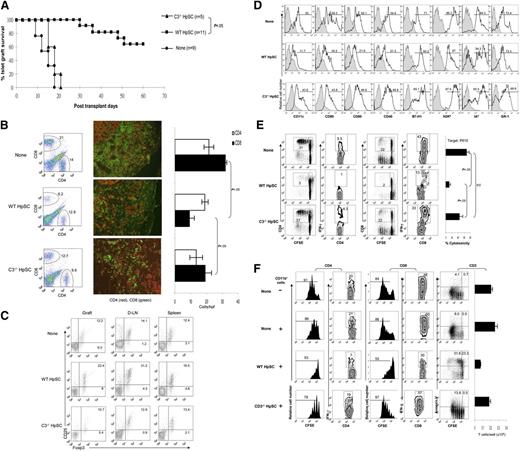
To determine the contribution of HpSC-produced C3 to immune regulation in vivo, islets isolated from BALB/c mice were mixed with the HpSCs from C3−/− or WT (both B6) mice, and transplanted under the renal capsule of diabetic (streptozotocin-induced) B6 recipients. As previously demonstrated,10-12 cotransplantation with WT HpSCs resulted in long-term survival in ∼60% of islet allografts, while all islet allografts that were cotransplanted with C3−/− HpSCs were rejected within 21 days (Figure 2A, P < .05 vs the WT HpSC group). HpSCs deficient in C3 largely lost their ability to protect islet allografts, suggesting a crucial role of C3 produced by HpSCs in modulating the immune response. To understand the underlying mechanisms through which C3 from HpSCs play a role in protecting islet allografts, graft-infiltrating T cells were isolated on POD 7 and analyzed by both flow cytometry and immunohistochemistry. Cotransplantation with WT HpSCs was associated with a reduced frequency of CD8+ T cells compared with the islet-alone grafts. The reduction of CD8+ T cells was significantly reversed in the C3−/− HpSC group (Figure 2B left panels). These changes were confirmed by immunohistochemical staining for graft-infiltrating CD8+ T cells and showed that HpSCs deficient in C3 lost their ability to inhibit the CD8+ T-cell response in islet allografts (Figure 2B middle and right panels), suggesting a critical role for C3 produced by HpSCs in regulating the effector T-cell response. To determine whether C3 was affecting Treg cell development and function, T cells isolated from the islet allografts, draining lymph nodes (D-LNs), and spleen were analyzed by flow analysis following staining for CD25 and Foxp3 (gated on CD4+ cells). In contrast to cotransplantation with WT HpSCs, which resulted in enhanced frequencies of CD25+Foxp3+ T cells in all tested compartments (particularly in grafts and D-LNs), cotransplantation with C3−/− HpSCs failed to increase the frequency of Treg cells (Figure 2C), indicating that HpSC-produced C3 participates in expansion of Treg cells.
Impact of HpSC-produced C3 on induction of MDSCs in vivo. Three hundred BALB/c islets were mixed with 2.5 × 105 HpSCs isolated from C3−/− or WT (B6) mice and transplanted into diabetic B6 recipients. Islet allografts alone served as controls (None). Islet graft survival was monitored by blood glucose levels as described in “Materials and methods.” For cellular analyses, the animals were sacrificed on POD 7. (A) HpSCs deficient in C3 lost their ability to protect islet allografts. (B) C3 deficiency in cotransplanted HpSC reversed attenuation of CD8+ T-cell infiltration in the islet allografts. The infiltrating cells were isolated from the islet allografts, and analyzed for CD4+ and CD8+ T cells by flow cytometry (left panels, the number is the percentage of positive cells in the total cell population) and immunohistochemistry. The positive cells were counted under a microscope. A total of 10 hpf’s were randomly selected in each graft and expressed as mean cells per hpf ± 1 SD (middle and right panels). (C) Fewer Treg cells are induced by cotransplanted C3−/− HpSCs. T cells isolated from islet grafts, D-LNs, and spleen were stained for CD4, CD25, and Foxp3, and analyzed by flow cytometry by gating on CD4+ T cells. The number is the percentage of positive cells in the CD4+ cell population. (D) Impact of HpSCs produced C3 on key molecule expression on myeloid cells. CD11b+ cells purified from islet allografts were analyzed for expression of the indicated surface markers by flow cytometry and expressed as histograms. The filled areas are isotype controls. The number is the percentage of positive cells in the CD11b+ cell population. (E) Myeloid cells isolated from islet/C3−/− HpSC grafts demonstrate high allostimulatory activity. CFSE-labeled B6 spleen T cells were cultured for 3 days with the irradiated CD11b+ cells isolated from the islet grafts in each group (at a ratio of 10:1). T-cell responses were determined by CFSE dilution and intracellular expression of IFN-γ in CD4 and CD8 T cells. The number is the percentage of positive cells in the T-cell subset. (Right panel) Generation of CTLs. B6 spleen T cells cultured, at a ratio of 10:1, for 5 days, with CD11b+ cells isolated from islet grafts that had been pulsed with BALB/c spleen cell lysates were used as effectors. P815 (H2d), EL4 (H2b), or R1.1 (H2k) lymphoma cells labeled with the fluorescent dye BCECF were used as donor-specific, syngeneic, and third-party targets, respectively, and expressed as a percentage of H2d-specific cytotoxicity ± 1 SD (n = 3). No cytotoxicity was generated against syngeneic and third-party targets (data not shown). (F) Impact of HpSC-produced C3 on differentiation of suppressive myeloid cells. CD11b+ cells isolated from islet grafts from each group were added into CFSE-labeled BALB/c spleen T-cell (2 × 105) culture (at a ratio of 1:10) in which T-cell responses were elicited by addition of anti-CD3 mAb (2 μg/mL) for 3 days. No addition of CD11b+ cells served as control. CD4+ or CD8+ T-cell responses were determined by CFSE dilution (proliferation) and intracellular staining for IFN-γ. Expression of annexin V was analyzed on T-cell (CD3+) populations, and T-cell absolute numbers were calculated based on flow analysis data (right panel). The number is the percentage of positive cells in the T-cell subset or whole T cells. The data are representative of three separate experiments.
Impact of HpSC-produced C3 on induction of MDSCs in vivo. Three hundred BALB/c islets were mixed with 2.5 × 105 HpSCs isolated from C3−/− or WT (B6) mice and transplanted into diabetic B6 recipients. Islet allografts alone served as controls (None). Islet graft survival was monitored by blood glucose levels as described in “Materials and methods.” For cellular analyses, the animals were sacrificed on POD 7. (A) HpSCs deficient in C3 lost their ability to protect islet allografts. (B) C3 deficiency in cotransplanted HpSC reversed attenuation of CD8+ T-cell infiltration in the islet allografts. The infiltrating cells were isolated from the islet allografts, and analyzed for CD4+ and CD8+ T cells by flow cytometry (left panels, the number is the percentage of positive cells in the total cell population) and immunohistochemistry. The positive cells were counted under a microscope. A total of 10 hpf’s were randomly selected in each graft and expressed as mean cells per hpf ± 1 SD (middle and right panels). (C) Fewer Treg cells are induced by cotransplanted C3−/− HpSCs. T cells isolated from islet grafts, D-LNs, and spleen were stained for CD4, CD25, and Foxp3, and analyzed by flow cytometry by gating on CD4+ T cells. The number is the percentage of positive cells in the CD4+ cell population. (D) Impact of HpSCs produced C3 on key molecule expression on myeloid cells. CD11b+ cells purified from islet allografts were analyzed for expression of the indicated surface markers by flow cytometry and expressed as histograms. The filled areas are isotype controls. The number is the percentage of positive cells in the CD11b+ cell population. (E) Myeloid cells isolated from islet/C3−/− HpSC grafts demonstrate high allostimulatory activity. CFSE-labeled B6 spleen T cells were cultured for 3 days with the irradiated CD11b+ cells isolated from the islet grafts in each group (at a ratio of 10:1). T-cell responses were determined by CFSE dilution and intracellular expression of IFN-γ in CD4 and CD8 T cells. The number is the percentage of positive cells in the T-cell subset. (Right panel) Generation of CTLs. B6 spleen T cells cultured, at a ratio of 10:1, for 5 days, with CD11b+ cells isolated from islet grafts that had been pulsed with BALB/c spleen cell lysates were used as effectors. P815 (H2d), EL4 (H2b), or R1.1 (H2k) lymphoma cells labeled with the fluorescent dye BCECF were used as donor-specific, syngeneic, and third-party targets, respectively, and expressed as a percentage of H2d-specific cytotoxicity ± 1 SD (n = 3). No cytotoxicity was generated against syngeneic and third-party targets (data not shown). (F) Impact of HpSC-produced C3 on differentiation of suppressive myeloid cells. CD11b+ cells isolated from islet grafts from each group were added into CFSE-labeled BALB/c spleen T-cell (2 × 105) culture (at a ratio of 1:10) in which T-cell responses were elicited by addition of anti-CD3 mAb (2 μg/mL) for 3 days. No addition of CD11b+ cells served as control. CD4+ or CD8+ T-cell responses were determined by CFSE dilution (proliferation) and intracellular staining for IFN-γ. Expression of annexin V was analyzed on T-cell (CD3+) populations, and T-cell absolute numbers were calculated based on flow analysis data (right panel). The number is the percentage of positive cells in the T-cell subset or whole T cells. The data are representative of three separate experiments.
HpSCs deficient in C3 are impaired in their ability to induce MDSCs in vivo
To determine the effect of HpSC-produced C3 on differentiation of myeloid cells in vivo, the myeloid (CD11b+) cells were isolated from the islet/C3−/− HpSC grafts on POD 7, as previously described,11 ,12 and analyzed for expression of CD11c and other key surface molecules, as well as their effect on T cell response. The results were compared with the CD11b+ cells isolated from islet alone or islet/WT HpSC grafts. As shown in Figure 2D, in islet/WT HpSC grafts, the myeloid cells contained significantly fewer DCs (CD11c+ cells 11.7%, compared with 55% in islet-alone grafts) but greater numbers of CD11c− cells (90%) and exhibited an immature phenotype (low expression of CD40, CD80 and CD86). These changes were reversed in the islet/C3−/− HpSC grafts (Figure 2D), suggesting a role for C3 produced by HpSCs in inhibiting DC differentiation and promoting the generation of MDSCs. The allostimulatory activity of the CD11b+ cells isolated from the grafts was examined in MLR and cytotoxic T-lymphocyte (CTL) generation assays. The proliferation of T cells was stimulated by isolated and irradiated CD11b+ cells pulsed with BALB/c spleen cell lysates. The CD11b+ cells in islet/WT HpSC grafts elicited lower T-cell proliferative response with low IFN-γ production, and generated low specific CTL activity, compared with no HpSC control, while the CD11b+ cells isolated from islet/C3−/− HpSC grafts demonstrated strong allostimulatory activities in both MLR and CTL assays (Figure 2E). To test the immune inhibitory activity, the isolated CD11b+ cells were added into the culture of T cells (at a ratio of 1:10). Proliferation of the T cells was induced by anti-CD3 mAb. Compared with CD11b+ cells from islet-alone grafts, CD11b+ cells from islet/WT HpSC grafts suppressed proliferative responses and IFN-γ production in both CD4+ and CD8+ T cells, with reduction of T-cell numbers resulting from enhanced T-cell apoptosis. These changes were markedly reversed in CD11b+ cells isolated from islet/C3−/− HpSC grafts (Figure 2F), demonstrating an impairment of immune-inhibitory activity in C3−/− HpSCs. Taken together, these in vivo data indicate that C3 produced by HpSCs is required to promote generation of MDSCs.
iC3b participates in generation of MDSCs
Since C3 is mainly produced by hepatocytes, why is the development of myeloid cells preferentially impacted by HpSCs produced C3? To exclude the possibility that C3 of HpSCs is modified through alternative splicing and/or posttranslational modifications, producing the isoforms different from those produced by hepatocytes, cDNAs of C3 from HpSCs and hepatocytes were synthesized and compared with GenBank. No differences were found between hepatic and HpSC-derived C3 (supplemental Figure 2A), suggesting no evidence of alternative splicing. Western blotting analysis showed that the molecular weight of the C3 produced by HpSCs was the same as that produced by hepatocytes (supplemental Figure 2B), indicating no posttranslational modifications.
It was recently demonstrated that C3 activation product iC3b plays an important role in the induction of immune tolerance in the anterior chamber of the eye via ligation to complement receptor type 3 (CR3) on antigen-presenting cells (APC) and results in the sequential production of transforming growth factor-β (TGF-β) and interleukin-10 (IL-10).22 To examine the impact of HpSCs on the activation and generation of C3 activation products, HpSCs were added into the DC culture and analyzed by flow cytometry to detect the deposition of C3 activation products on the CD11b+ cells using anti-C3 Ab. C3 and its activation products share the same Ab-binding epitope. However, C3, unlike its activation products, does not bind to the cell surface.23 The data showed that addition of HpSCs led to markedly upregulated anti-C3 staining on the CD11b+ cell surface (Figure 3A), indicating that HpSCs promote the binding of C3 activation products on myeloid cells. C3 is activated through 1 of 3 activation pathways, among which the alternative pathway is the simplest, occurs spontaneously, and is dependent on factor B and factor D. To determine whether HpSCs produce functional factor B and factor D, a classical zymosan-based complement activation alternative pathway assay was utilized using sera from factor B−/− or factor D−/− mice as described in “Materials and methods.” HpSC culture medium well compensated for the deficiency of factor B and factor D in the serum (Figure 3B left and middle panels), indicating that HpSCs produce functional factor B and factor D, which can activate C3 through the alternative pathway. This was supported by PCR analysis results showing that HpSC expressed both factor B and factor D mRNA (Figure 3B right panels).
The role of iC3b on MDSC differentiation. (A) HpSCs enhances accumulation of C3 activation products on myeloid cells. HpSCs isolated from B6 mice were added at the beginning of B6 BM cell culture at a ratio of 1:80 in the presence of GM-CSF for 5 days. Culture without HpSCs served as the control (None). CD11b+ cells were purified with magnetic beads, stained with anti-C3 for flow analysis, and expressed as a histogram (the filled area indicates the isotype control). (B) HpSCs produce functional factor B and D. Flow analysis of C3 activation product deposition on zymosan that were incubated with sera from factor B or factor D knockout mice with compensation by control medium (Control) or HpSC culture medium. (Right panels) mRNA expression of factor B and D in HpSCs determined by semiquantitative PCR. (C) Exogenous iC3b facilitated generation of CD11b+CD11c− cells in a dose-dependent manner. Graded doses (as indicated) of exogenous iC3b or C3d were added at the beginning into B6 BM cell culture with GM-CSF for 5 days. The floating cells were harvested and stained with anti-CD11b and -CD11c and analyzed by flow cytometry (gated on CD11b+ cells). The number is the percentage of positive staining in CD11b+ cells. The expression of CD11c was also demonstrated in a histogram (gated on CD11b+ cells) following incubation with various concentrations of iC3b (right panel). (D) The iC3b-conditioned myeloid cells show immature phenotype. Expression of the key molecules was analyzed by flow cytometry by gating on CD11b+ cells and expressed as histograms (open area refers to the isotype control). The figure shows the results of iC3b (10 μg/mL) group compared with no iC3b control. (E) The iC3b-induced myeloid cells inhibit the T-cell proliferative response. CFSE-labeled BALB/c spleen T cells were elicited with B6 DCs at a ratio of 20:1 (3 days). The tested CD11b+ cells were purified from the iC3b (10 μg/mL) group, and added at the beginning into the culture at a DC:CD11b+ cell ratio of 1:0.5 or 1:1. Addition of DCs instead of the CD11b+ cells at a ratio of 1:1 served as control. The proliferation of T cells was determined by CFSE dilution (gated on the CD3+ population). These data are representative of three separate experiments.
The role of iC3b on MDSC differentiation. (A) HpSCs enhances accumulation of C3 activation products on myeloid cells. HpSCs isolated from B6 mice were added at the beginning of B6 BM cell culture at a ratio of 1:80 in the presence of GM-CSF for 5 days. Culture without HpSCs served as the control (None). CD11b+ cells were purified with magnetic beads, stained with anti-C3 for flow analysis, and expressed as a histogram (the filled area indicates the isotype control). (B) HpSCs produce functional factor B and D. Flow analysis of C3 activation product deposition on zymosan that were incubated with sera from factor B or factor D knockout mice with compensation by control medium (Control) or HpSC culture medium. (Right panels) mRNA expression of factor B and D in HpSCs determined by semiquantitative PCR. (C) Exogenous iC3b facilitated generation of CD11b+CD11c− cells in a dose-dependent manner. Graded doses (as indicated) of exogenous iC3b or C3d were added at the beginning into B6 BM cell culture with GM-CSF for 5 days. The floating cells were harvested and stained with anti-CD11b and -CD11c and analyzed by flow cytometry (gated on CD11b+ cells). The number is the percentage of positive staining in CD11b+ cells. The expression of CD11c was also demonstrated in a histogram (gated on CD11b+ cells) following incubation with various concentrations of iC3b (right panel). (D) The iC3b-conditioned myeloid cells show immature phenotype. Expression of the key molecules was analyzed by flow cytometry by gating on CD11b+ cells and expressed as histograms (open area refers to the isotype control). The figure shows the results of iC3b (10 μg/mL) group compared with no iC3b control. (E) The iC3b-induced myeloid cells inhibit the T-cell proliferative response. CFSE-labeled BALB/c spleen T cells were elicited with B6 DCs at a ratio of 20:1 (3 days). The tested CD11b+ cells were purified from the iC3b (10 μg/mL) group, and added at the beginning into the culture at a DC:CD11b+ cell ratio of 1:0.5 or 1:1. Addition of DCs instead of the CD11b+ cells at a ratio of 1:1 served as control. The proliferation of T cells was determined by CFSE dilution (gated on the CD3+ population). These data are representative of three separate experiments.
To examine whether iC3b or C3d participates in the generation of MDSCs, various amounts of exogenous iC3b or C3d were added into DC culture. The presence of exogenous iC3b inhibited generation of DCs (CD11c+), but promoted differentiation of CD11b+CD11c− cells in a dose-dependent manner. However, addition of C3d did not affect expression of CD11c in myeloid cells (Figure 3C), suggesting that iC3b, but not C3d, plays a role in development of myeloid cells with suppressive function. iC3b-conditioned CD11b+ cells demonstrated immature phenotype (low CD40, CD86, and major histocompatibility complex II (Figure 3D) and the addition of iC3b-CD11b+ cells markedly inhibited the alloreactive T-cell proliferative response in a dose-related manner. This was not due to overcrowding of cells because addition of the same amounts of DCs (instead of iC3b-CD11b+ cells) did not suppress the T-cell proliferative response (Figure 3E). These observations indicate a crucial role for iC3b in promoting the differentiation of MDSCs.
Discussion
The expansion of MDSCs has been described in cancers and is believed to be regulated by factors produced by tumor cells.24 While studying the underlying mechanisms of liver transplantation tolerance in mice, we discovered that the liver stromal cell, HpSC, is immensely immunosuppressive and can effectively protect islet allografts when cotransplanted under the renal capsule.10 Islet allograft protection can be partially attributed to the accumulation of MDSCs in the grafts.11 ,17 It is not surprising that the liver contains cells favoring induction of immune tolerance. Due to the anatomical location, the liver is continuously exposed to various antigens, including dietary and commensal protein, and the liver has acquired the ability to regulate immune responses to those harmless antigens over time.
HpSCs produce VEGF, GM-CSF, G-CSF, and IL-6 upon activation.9 ,25 These factors enhance expansion of MDSCs in cancers by promoting myelopoiesis and inhibiting maturation of myeloid cells.26-30 However, induction of MDSCs by HpSCs appears unlikely to be mediated by these factors because HpSCs from G-CSF or GM-CSF knockout mice retained the ability to induce MDSCs. The HpSCs treated with specific small interfering RNA to silence VEGF production are still capable of promoting MDSCs.12 The results suggest that HpSCs and tumor cells induce MDSCs through different pathways.
Analysis of the HpSC culture supernatant fractions suggested the involvement of C3 in the promotion of MDSC differentiation.12 We demonstrate here that protection of islet allografts by HpSC is critically dependent on C3 expression, since cotransplantation with C3−/− HpSCs led to prompt rejection of islet allografts, suggesting C3 produced by HpSCs is an important contributor to inhibition of immune response. Although these observations are contradictory to the prevailing concept that the function of complement, which is known to link the innate immune response to the subsequently activated adaptive immunity,31 accumulating evidence suggests that complement proteins have a negative regulatory effect on immune response. Several studies report the link between complement deficiency and the development of autoimmune diseases.32 ,33 Induction of tolerance by intraocular (immune privilege site) injection of antigens also requires C3.22 Activation of CD4+ T cells with CD3 and complement regulator protein CD46 induces IL-10 producing Treg 1 cells.34 ,35 Furthermore, mice lacking C3 showed accelerated rejection of minor H disparate skin grafts and resistance to induction of tolerance.36 The underlying mechanisms are not completely understood. We demonstrate in this study that rejection of the islet allografts cotransplanted with C3−/− HpSC is associated with reduced accumulation of MDSC, suggesting a critical role for HpSC derived C3 in the induction of MDSCs. This finding was supported in vitro by adding C3−/− HpSC into BM-derived DC culture; deficiency in C3 in HpSCs markedly attenuated their ability to inhibit development of DCs and promote MDSCs, indicating a requirement of C3 in induction of MDSCs.
C3 is a central component in the complement system. It is mainly produced by hepatocytes and exists abundantly in the serum.37 We wondered why the effect of HpSCs on induction of MDSCs is significantly impaired if HpSC are deficient in expression of C3. One possibility is that HpSCs may produce a C3 isoform that is structurally and functionally different from that produced by hepatocytes. However, we did not find any evidence of alternative slicing or posttranslational modifications occurring in C3 produced by HpSCs in this study. Instead, we found that the presence of HpSCs enhanced binding of C3 activation products on cocultured myeloid cells, suggesting that HpSC facilitated the generation of C3 activation products. C3 undergoes 3 activation pathways (classical, lectin, and alternative), among which the alternative pathway is the simplest, occurs spontaneously, and requires factor B and factor D.40 HpSCs are well equipped with factors B and D and facilitate the accumulation of C3 activation products on cocultured myeloid cells. We showed that iC3b, but not C3d, can partially substitute the effect of HpSCs on induction of MDSCs. iC3b has been shown to be involved in the regulation of the immune response. Thus, induction of tolerance by intraocular injection of antigens is mediated by iC3b that binds to APC and arrests their maturation.22 iC3b released from apoptotic tumor cells induces immune tolerance by binding to APC.39 The binding of iC3b to CR3 on DCs stimulates the production of TGF-β and IL-10 and promotes immune tolerance.22 Here, we provide evidence that iC3b is an important factor in facilitating the expansion of MDSCs. Addition of exogenous iC3b into BM-derived DC culture markedly inhibited the development of CD11c+ DCs and enhanced the differentiation of myeloid cells with potent T-cell suppressive capacity (Figure 3D-E). It is well known that iC3b can be recognized by various complement receptors CR1, CR2, CR3, and CR4.40 CR2 (CD21) is found on B cells. CR3 (CD11b/CD18) is expressed on myeloid cells and phagocytes.41 Our laboratory is now working on illustrating the role of these receptors in induction of MDSCs by iC3b, although we cannot exclude the role of the C3 products other than iC3b. Thus, C3a has its conventional role in innate immunity by binding to its receptor C3aR to activate various signaling pathways, including the mitogen-activated protein kinase/nuclear factor-κB pathway.42 ,43
We and others have recently demonstrated that ligation of C3aR is also integrally involved in the regulation of the T-cell response44 ,45 and osteoclast differentiation.46 Interestingly, in these circumstances, as well as in the experimental settings used in this study, the effector complement components are locally produced by related cells, not systemic complement components produced by hepatocytes, which is most likely due to the relatively high concentrations of complement in the local environment outside of the vasculature. Local production and activation of complement have been shown to play a role in T-cell activation and generation of Th1 responses.37 ,47 The local environment theory can explain why HpSC/islet cotransplanted under the renal capsule are protected, but C3−/− HpSC/islet cotransplants are rejected. In HpSC/islet cotransplants, the infiltrating myeloid cells which are in close proximity to HpSCs can be regulated and differentiated into MDSCs by local iC3b derived from HpSCs, while there is insufficient iC3b accumulation surrounding the infiltrating myeloid cells in C3−/− HpSC/islet cotransplants although other cells in the kidney can also produce C3.
The dual effects of complement on immune response have been well documented. Compared with WT controls, C3−/− macrophages showed a reduced capability to elicit alloreactive T-cell response, and graft-derived complement is required for priming alloreactive T cells.48 ,49 Tumor-driven complement activation attributes set up a local immunosuppressive environment to promote tumor growth,50 suggesting an essential role of C3 produced by the local compartment in T-cell activation. However, our results demonstrated suppressive activities of C3 derived from cotransplanted HpSCs on myeloid cell differentiation. The contradictory effect of C3 on immune response may be due to other coexisting factors or cell populations in the local inflammatory environment, which could directly or indirectly modulate C3 signaling on immune cells. We demonstrated in this study that HpSC deficient in C3 did not completely lose their capacity to induce MDSCs, which suggests the involvement of other factors that may synergize with C3 to promote MDSC differentiation. A recent study reported immune regulatory activities of other C3 activation products. C3b, the main component of C5 convertase, is responsible for cleaving C5 to produce C5a and C5b. Generation of C5a in tumors enhanced tumor growth by suppressing the antitumor CD8+ T-cell response, which was associated with the recruitment of MDSCs into tumors.45 Elucidating the cellular and molecular mechanisms mediating the immunomodulatory activity of HpSCs will provide more insight into the inherent tolerogenicity of the liver and be of value in the design of novel therapeutic approaches for treatment of transplantation rejection and autoimmune diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Kathleen Brown for technical support.
This work was supported by National Institutes of Health grants DK084192 (L.L.) and AI090468 (S.Q.).
C.-C.H. was a research fellow from Department of Surgery, Chang Gung Memorial Hospital, Chang Gung University Medical School, Chia-Yi, Taiwan. H.-S.C. was research fellow from Department of General Surgery, Chang-Gung Memorial Hospital, Linkou, Chang-Gung University Medical School, Taoyuan, Taiwan.
National Institutes of Health
Authorship
Contribution: C.-C.H. participated in research design, performed most of the in vitro experiments, analyzed the data, and wrote the manuscript; H.-S.C. and H.-R.Y. performed most of the in vivo studies and data analysis; F.L. performed the complement activation assay and served as a senior consultant; L.W. performed qPCR; J.Q. performed the C3 dose-dependent study; S.B. participated in manuscript writing; J.J.F. served as a senior consultant and contributed to experimental design; and S.Q. and L.L. contributed to the research design and preparation of the manuscript, and served as the sponsors of the project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lina Lu, Department of Immunology, Lerner Research Institute, Cleveland Clinic, 9500 Euclid Ave, NE60, Cleveland, OH 44195; e-mail: lul2@ccf.org; and Shiguang Qian, Department of Immunology, Lerner Research Institute, Cleveland Clinic, 9500 Euclid Ave, NE60, Cleveland, OH 44195; e-mail: qians@ccf.org.
References
Author notes
C.-C.H. and H.-S.C. contributed equally to this work.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal