Key Points
IRF8 induces the Klf4 gene in myeloid progenitors; this transcription factor cascade is essential for Ly6C+ monocyte development.
IRF8 binding to genomic targets promotes H3K4me1, a chromatin signature for promoter-distal enhancers, thereby inducing gene expression.
Abstract
Monocytes regulate host defenses, inflammation, and tissue homeostasis. The transcription factor interferon regulatory factor-8 (IRF8) stimulates monocyte/macrophage differentiation, yet genome-wide understanding of the differentiation program initiated by IRF8 is lacking. By combining chromatin immunoprecipitation sequencing with gene expression profiling, we show that during IRF8-dependent monocyte differentiation, IRF8 binding occurs at both promoter-proximal and promotor-distal regions together with the transcription factor PU.1 and is associated with gene induction. Many of the promoter-distal IRF8 binding sites show an increase in histone H3 lysine 4 monomethylation, a signature for enhancers. However, about half the IRF8-induced genes were not bound by IRF8, suggesting the involvement of downstream transcription factors. Analysis of DNA motifs in cis-regulatory elements of these indirect IRF8 target genes predicted that Krüppel-like factor-4 (KLF4)—essential for Ly6C+ monocyte development—is one such factor. Indeed, monocyte development in Irf8−/− mice is as defective as that in Klf4−/− chimeric mice. Moreover, Irf8−/− monocyte-dendritic cell progenitors do not express Klf4 messenger RNA. Introduction of KLF4 into an Irf8−/− myeloid progenitor cell line induced a subset of IRF8 target genes and caused partial monocyte differentiation. Taken together, our present results uncover genome-wide behavior of IRF8 and identify an IRF8-KLF4 axis that operates during monocyte differentiation.
Introduction
Monocytes play critical roles in inflammation, innate immune responses, and tissue homeostasis.1 Although they were once considered simply an intermediate between bone marrow progenitors and tissue macrophages, it is now commonly accepted that monocytes also have effector and regulatory functions.
Functionally distinct subsets of monocytes have been reported in humans, mice, and other species.2 In mice, subsets of monocytes are distinguished by their expression of Ly6C. Ly6C+ inflammatory monocytes are selectively recruited to inflamed tissues and lymph nodes and produce large amounts of inflammatory cytokines. Furthermore, Ly6C+ monocytes differentiate into M1-type macrophages that promote interleukin-12–mediated Th1-type responses or into a subset of dendritic cells (DCs) that produce tumor necrosis factor-α and inducible nitric oxide synthase (known as TipDCs). Conversely, Ly6C− patrolling monocytes crawl on the luminal surfaces of blood vessels, are implicated in scavenging dead cells and toxic compounds, and differentiate into M2-type macrophages. M2 macrophages preferentially stimulate Th2 responses and are thought to be important for tissue repair and resolution of inflammation. Under homeostatic conditions, Ly6C– monocytes may give rise to alveolar macrophages.1,3
Monocytes originate in the bone marrow and circulate in the blood, bone marrow, and spleen. Hematopoietic stem cells give rise to myeloid progenitors,4 which pass through common myeloid progenitors, granulocyte-monocyte progenitors, and monocyte-dendritic cell progenitors (MDPs) in the bone marrow.1,5 MDPs, defined as lineage markers (Lin)− CD115+ CD117int/+ cells, give rise to monocytes,6,7 although how the monocyte subsets are generated from MDPs remains controversial. Although it has been reported that Ly6C− monocytes are derived from Ly6C+ monocytes,8 it is presumed that Ly6C− monocytes might also originate by direct differentiation from bone marrow progenitors.6
Coordinated regulation of gene expression by transcription factors is essential for the differentiation of immune cells.9,10 Gene knockout studies have shown several transcription factors to be essential for monocyte and macrophage differentiation. PU.1, a member of the ETS family transcription factors, is necessary for the earliest steps of myeloid and lymphoid lineage commitment, and its deficiency results in loss of monocytes, granulocytes, and B cells.4,11 Mice transplanted with Krüppel-like factor-4 (KLF4)-deficient fetal liver cells (Klf4−/− chimera mice) have fewer monocytes, especially the Ly6C+ subset.12 In contrast, the nuclear orphan receptor NR4A1 is required for the generation of Ly6C− monocytes but not Ly6C+ monocytes.6 The transcription factors MAFB, c-MAF, and EGR1 are also known to promote monocyte/macrophage differentiation.13-15
Interferon regulatory factor-8 (IRF8), a hematopoietic cell-specific member of the IRF family, directs myeloid progenitor cells to differentiate into macrophages.16 However, its role in the development of monocyte subsets has yet to be clarified. It has been recognized that IRF8 functions as either a transcriptional activator or repressor, depending on the formation of different heterodimeric complexes with partner molecules and target DNA elements.17,18 IRF8 binds to the interferon-stimulated response element (ISRE; A/G NGAAANNGAAACT) in association with IRF1 or IRF2 to repress gene expression. Conversely, the IRF8-PU.1 heterodimer leads to transcriptional activation of genes containing the ETS-IRF composite element (EICE; GGAANNGAAA), or the IRF-ETS composite sequence (IECS; GAAANN[N]GGAA).19,20 Irf8−/− mice lack bone marrow resident macrophages, CD8α+ DCs, and plasmacytoid DCs in lymphoid organs, and nonlymphoid tissue CD103+ DCs.21-25 Indeed, IRF8 mutations were associated with human DC immunodeficiency.26 In contrast, the numbers of neutrophils and osteoclasts are dramatically increased in Irf8−/− mice.27,28
Given that Irf8−/− mice display a broad range of abnormalities in terms of myeloid cell development, IRF8 may function at an early step of the transcriptional program that governs differentiation from myeloid progenitors to monocytes/macrophages. However, our understanding of how IRF8 regulates myeloid differentiation on a genome-wide scale remains incomplete. Previous ChIP-on-chip (chromatin immunoprecipitation followed by microarray hybridization) studies of IRF8 have used differentiation-arrested monocytic cell lines or mouse lungs infected with pathogen but not cells undergoing monocytic differentiation.29,30 It was recently revealed that distal enhancers, characterized by histone H3 lysine 4 monomethylation (H3K4me1), are critical for cell lineage specification.31 However, the above-mentioned ChIP-on-chip studies examined the regions relatively proximal (−7.5 kb to +2.5 kb) to the transcription start sites (TSSs) and did not examine epigenetic changes.
In this study, we combined ChIP sequencing (ChIP-seq) and gene expression profiling to show that promoter-distal binding of IRF8 induces H3K4me1. We further identified the IRF8-KLF4 axis as a critical component of the monocyte differentiation program and demonstrated that Irf8−/− mice lack Ly6C+ monocytes and have much lower Ly6C− monocyte counts.
Methods
Full details on retroviral transduction, generation of bone marrow chimeras, flow cytometric analysis and cell isolation, microarray, gene ontology analysis, ChIP-seq data analysis, qPCR with reverse transcription (qRT-PCR), western blotting, and peritoneal exudate cells are provided in supplemental Methods (available on the Blood Web site).
Mice and cells
Ly5.1, Ly5.2, and Irf8−/− mice in a C57BL/6 background were used at 7 to 9 weeks of age.27 All animal experiments were conducted in accordance with the Guidelines for Proper Conduct of Animal Experiments (Science Council of Japan), and all protocols were approved by the institutional review boards of Yokohama City University (Protocol #F11-85). An Irf8−/− myeloid progenitor cell line Tot2 was described previously.16
ChIP-seq and ChIP-polymerase chain reaction
ChIP assays were performed as described previously,20 with slight modifications. Briefly, the lysates of cross-linked Tot2 cells transduced with either empty murine stem-cell virus (MSCV) retroviral vector (MSCV-puro) or MSCV-IRF8-puro were sonicated to shear the genomic DNA into fragments of 150 to 500 bp. Immunoprecipitation was then performed by using 10 µg of goat anti-IRF8 antibody (C-19; Santa Cruz Biotechnology, Santa Cruz, CA), 2 µg of rabbit anti-PU.1 antibody (T-21; Santa Cruz Biotechnology), or 2 µg of rabbit anti-H3K4me1 antibody (Ab8895, Abcam, Cambridge, UK). Antibodies were crosslinked to magnetic Dynabeads-Protein G (Invitrogen, Carlsbad, CA). For the ChIP-seq procedure, purified ChIP DNA was modified by end repair and adapter ligation before separation by agarose gel electrophoresis to select DNA fragments between 170 and 230 bp. Gel-purifed DNA was amplified by polymerase chain reaction (PCR) for ChIP-Seq library preparation according to Illumina’s manuals (Illumina, San Diego, CA). After verifying the qualities by Bioanalyzer (Agilent, Palo Alto, CA), 8 pmol of each ChIP-Seq library was used directly for cluster generation on Cluster Station (Illumina). The sequencing analysis by Genome Analyzer IIx (Illumina) was performed according to the manufacturer’s manuals. In ChIP-PCR assays, purified ChIP DNA samples from three biological triplicates were used for quantification of the specific region of genomic DNA (80 to 200 bp) by duplicate quantitative PCR (qPCR) amplifications. Input DNA (0.1%) was used for normalization. The primers used for ChIP-PCR are described in supplemental Table 1.
Results
ChIP-seq analysis and gene expression profiling during IRF8-induced monocyte differentiation
Restoration of IRF8 expression in the Irf8−/− myeloid progenitor cell line (Tot2 cells) causes differentiation into growth-arrested, functional monocytes/macrophages in 6 days.16,21 This provides an ideal system for investigating the molecular mechanism of how IRF8 regulates myeloid development. Thus, we performed two genome-wide analyses for IRF8 at early phases of monocyte differentiation. The first involved ChIP-seq using an anti-IRF8 antibody (see supplemental Figures 1-3 and the next section for validation by ChIP-PCR), and the second microarray used to profile gene expression (the quality control of microarray data is shown in supplemental Figure 4). Whereas ChIP-seq identified 11 741 IRF8-bound genomic sites, gene expression profiling identified 2120 upregulated genes (fold change ≥ 2) and 1637 downregulated genes (fold change ≤ 0.5) in IRF8-transduced Tot2 cells compared with cells transduced with empty MSCV (supplemental Figure 5A and supplemental Tables 2-3).
Gene ontology (GO) analysis revealed that upregulated genes had annotations related to immunity (60.1%) and adhesion and cytoskelton (6.6%) (supplemental Figure 5B and supplemental Tables 4-5). Conversely, GO annotations related to metabolism (27.8%); cell cycle, proliferation, and survival (27.4%); and chromatin and transcription (21.8%) were overrepresented in the downregulated genes. Gene set enrichment analysis32 confirmed that induction of monocyte signature genes by IRF8 is highly significant (supplemental Figure 5C).
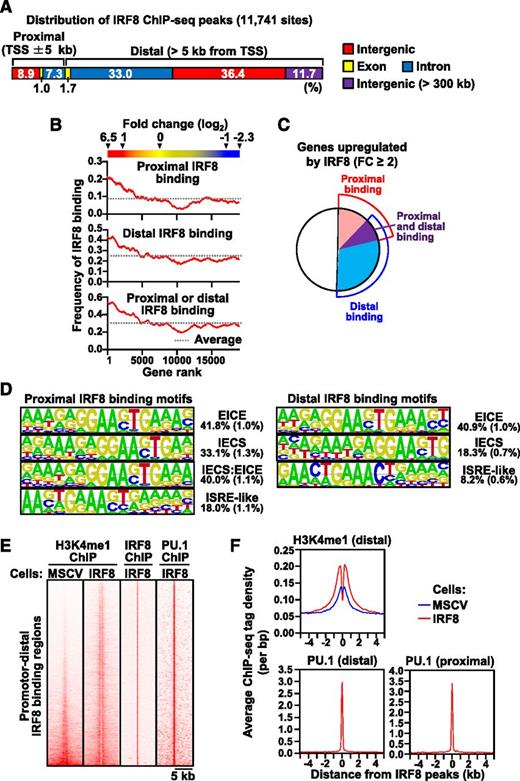
Approximately 17% of the IRF8 binding sites were located in the promoter-proximal region (±5 kb from TSSs) and 83% were located in the distal region (>5 kb from TSSs) (Figure 1A). This revealed for the first time that a majority of IRF8 binding occurs at distal intergenic or intragenic regions. Integrated analysis of the ChIP-seq data and the gene expression profiles showed that the frequencies of both proximal and distal (up to 300 kb) IRF8 binding were correlated with the level of gene induction (Figure 1B and supplemental Figure 6A). Furthermore, the numbers of IRF8 ChIP-seq peaks and tag counts per gene, which indicate the number of binding sites and the total amount of binding to the associated gene, respectively, were positively correlated with the magnitude of gene induction (supplemental Figure 6B-C), suggesting that IRF8 functions mainly as a transcriptional activator in differentiating monocytes. We also found that approximately half the IRF8-upregulated genes were bound by IRF8 (Figure 1C).
Genome-wide behavior of IRF8 during monocyte/macrophage differentiation. Tot2 cells were transduced with empty MSCV-puro or MSCV-IRF8-puro vectors. Transduced cells were selected by puromycin. (A) Summary of the locations of IRF8 ChIP-seq peaks in IRF8-transduced Tot2 cells. ChIP-seq for IRF8 was performed on day 3. (B) Correlation between IRF8 binding and gene expression changes. Gene expression profiling by microarray was performed in biological duplicates on day 4. Genes were ranked in order of fold induction by IRF8. The frequencies of the presence of promoter-proximal or -distal IRF8 ChIP-seq peak(s) per gene were calculated in a sliding window comprising 1000 ranked genes. (C) The frequencies of genes with promoter-proximal and/or -distal IRF8 binding among the IRF8-upregulated genes. FC indicates fold change. (D) De novo DNA motif analysis of promoter-proximal (left panel) and promotor-distal (right panel) IRF8 binding sites in IRF8-upregulated genes. Numbers indicate the percentage of the ChIP-seq peaks containing the motif. The background frequency is shown in parentheses. (E) Heatmaps of promoter-distal IRF8 binding, H3K4me1, and PU.1 binding. ChIP-seq analyses for H3K4me1 and PU.1 were performed on day 3. Each horizontal line represents the density of indicated ChIP-seq tags in the 10-kb region centered on the promoter-distal IRF8 peak summit. “Cells: MSCV” indicates MSCV-puro–transduced cells. “Cells: IRF8” indicates MSCV-IRF8-puro–transduced cells. (F) Cumulative H3K4me1 and PU.1 levels around IRF8 ChIP-seq peak positions. Histograms of averaged ChIP-seq tag densities are presented.
Genome-wide behavior of IRF8 during monocyte/macrophage differentiation. Tot2 cells were transduced with empty MSCV-puro or MSCV-IRF8-puro vectors. Transduced cells were selected by puromycin. (A) Summary of the locations of IRF8 ChIP-seq peaks in IRF8-transduced Tot2 cells. ChIP-seq for IRF8 was performed on day 3. (B) Correlation between IRF8 binding and gene expression changes. Gene expression profiling by microarray was performed in biological duplicates on day 4. Genes were ranked in order of fold induction by IRF8. The frequencies of the presence of promoter-proximal or -distal IRF8 ChIP-seq peak(s) per gene were calculated in a sliding window comprising 1000 ranked genes. (C) The frequencies of genes with promoter-proximal and/or -distal IRF8 binding among the IRF8-upregulated genes. FC indicates fold change. (D) De novo DNA motif analysis of promoter-proximal (left panel) and promotor-distal (right panel) IRF8 binding sites in IRF8-upregulated genes. Numbers indicate the percentage of the ChIP-seq peaks containing the motif. The background frequency is shown in parentheses. (E) Heatmaps of promoter-distal IRF8 binding, H3K4me1, and PU.1 binding. ChIP-seq analyses for H3K4me1 and PU.1 were performed on day 3. Each horizontal line represents the density of indicated ChIP-seq tags in the 10-kb region centered on the promoter-distal IRF8 peak summit. “Cells: MSCV” indicates MSCV-puro–transduced cells. “Cells: IRF8” indicates MSCV-IRF8-puro–transduced cells. (F) Cumulative H3K4me1 and PU.1 levels around IRF8 ChIP-seq peak positions. Histograms of averaged ChIP-seq tag densities are presented.
We next performed de novo motif analysis of the IRF8 binding sites of the upregulated genes. Regardless of whether they were proximal or distal to TSSs, IRF8-bound genomic sequences were enriched for the composite elements recognized by IRF and ETS (ie, EICE and IECS) (Figure 1D). This observation is consistent with the ability of IRF8 and PU.1 to form heterodimeric complexes in cells belonging to the monocyte/macrophage lineage.16 Interestingly, we also identified an IECS:EICE combined element (IRF-ETS-IRF) in the promoter-proximal IRF8 binding sites. ISRE-like motifs were also found in proximal and distal IRF8 binding sites, although these were poorly conserved and less abundant than the composite elements recognized by IRF and ETS.
Epigenetic studies have established H3K4me1 as a chromatin signature of enhancers.31 Given that IRF8 binds to promoter-distal regions and induces gene expression, we next tested whether H3K4me1 is enriched at the promoter-distal IRF8 binding sites. We chose to analyze distal regions to avoid detecting H3K4me1 as a byproduct of the initiation signature H3K4me3. We found that among the 8358 promoter-distal IRF8 binding regions, 7631 (91.3%) were positive for H3K4me1 (Figure 1E-F). Importantly, 3927 IRF8 binding regions (47.0%) were associated with increased H3K4me1 (fold change ≥ 2) compared with the same regions in empty MSCV-transduced IRF8-null Tot2 cells. The pattern of H3K4me1 at IRF8 binding regions showed a bimodal distribution more clearly than the corresponding regions in empty MSCV-transduced cells, which may be a sign of chromatin remodeling.
It has been reported that promoter-distal PU.1 binding initiates nucleosome remodeling followed by H3K4me1 in macrophages.33,34 In addition, the above de novo motif analysis as well as previous reports have suggested the cooperative binding of IRF and an ETS transcription factor such as PU.1. Thus, we next performed ChIP-seq analysis for PU.1 in IRF8-transduced Tot2 cells. The results showed that 71.9% of the promoter-distal IRF8 ChIP-seq peaks accompanied PU.1 peaks within 100 bp (Figure 1E-F). In addition, a majority (90.9%) of the distal H3K4me1 regions bound by IRF8 were co-bound by PU.1. ChIP-PCR results indicated that IRF8 binding leads to an increase in PU.1 binding on the peaks analyzed (supplemental Figure 3C), consistent with our previous report.20 Collectively, these results indicate that IRF8 binds to the distal enhancer regions along with PU.1. A majority (76.2%) of the promoter-proximal IRF8 ChIP-seq peaks also overlapped with PU.1 ChIP-seq peaks (Figure 1F).
KLF4 is a putative transcription factor downstream of IRF8
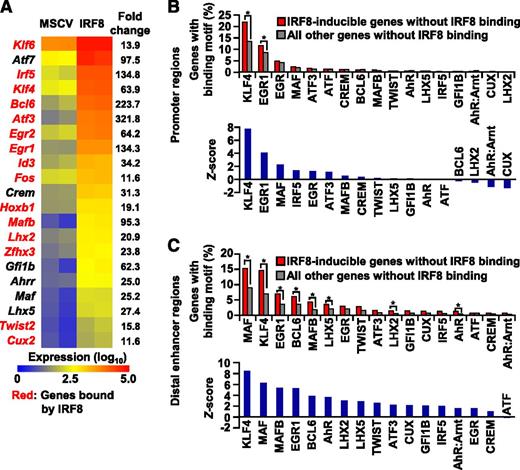
Nevertheless, IRF8 binding was not detected in the remaining half of the IRF8-induced genes (Figure 1C). This indicates that these “indirect” target genes may be regulated by another transcription factor(s), expression of which is induced by IRF8. Accordingly, the expression of 21 transcription factor genes, such as Klf6, Atf7, Irf5, and Klf4, was increased more than 10-fold by IRF8. Fourteen of these transcription factor genes were found to be bound by IRF8 at promoter-proximal and/or promotor-distal regions (Figure 2A, supplemental Table 6, and supplemental Figures 1-2). ChIP-PCR assays were also performed for 69 ChIP-seq peaks in biological triplicates (supplemental Figure 3). Among them, the binding was confirmed in 67 regions, validating the quality of our ChIP-seq data.
Transcription factors downstream of IRF8. (A) Expression levels of transcription factor genes upregulated by IRF8 more than 10-fold are displayed as a heatmap (also see supplemental Table 6). Genes bound by IRF8 are indicated in red. (B-C) Known DNA binding motif analysis for transcription factors in the 1-kb proximal regions upstream of TSSs (B) or the distal enhancer (H3K4me1) regions (C) of indirect IRF8 target genes or all other genes not bound by IRF8. The upper panels indicate the frequency of genes with these motifs. (*) P < .05 (χ2 test). The lower panels indicate the Z-score.
Transcription factors downstream of IRF8. (A) Expression levels of transcription factor genes upregulated by IRF8 more than 10-fold are displayed as a heatmap (also see supplemental Table 6). Genes bound by IRF8 are indicated in red. (B-C) Known DNA binding motif analysis for transcription factors in the 1-kb proximal regions upstream of TSSs (B) or the distal enhancer (H3K4me1) regions (C) of indirect IRF8 target genes or all other genes not bound by IRF8. The upper panels indicate the frequency of genes with these motifs. (*) P < .05 (χ2 test). The lower panels indicate the Z-score.
To identify transcription factors that act downstream of IRF8 and critically regulate the development of monocytes/macrophages, we evaluated the probability that cis-regulatory regions of the indirect IRF8 target genes contain the binding motif of each induced transcription factor. The cis-regulatory regions analyzed were promoter regions (1 kb upstream of TSSs) and distal H3K4me1-enriched enhancer regions. Whereas KLF4- and EGR1-binding motifs were found to be significantly overrepresented in the promoter regions, the binding motifs of MAF, KLF4, EGR1, BCL6, MAFB, LHX5, LHX2, and AhR were significantly enriched in the distal enhancer regions (Figure 2B-C). In particular, the KLF4 binding motif had the highest Z-score in both promoters and distal regions. From these results, we predicted that KLF4 was the transcription factor most likely to play a major role downstream of IRF8 during the development of monocytes/macrophages.
Klf4 is a direct target gene of IRF8
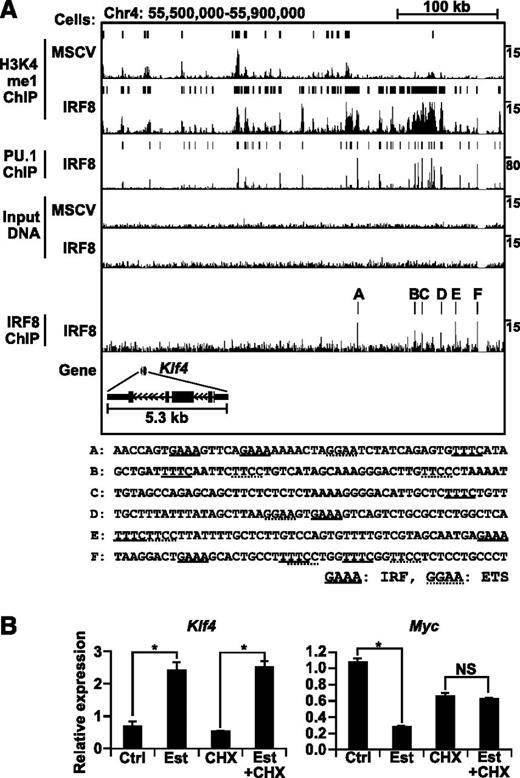
The ChIP-seq data at the Klf4 gene locus revealed a distal region with multiple IRF8 ChIP-seq peaks spanning 120 kb (208 to 328 kb upstream of the Klf4 TSS) (Figure 3A and supplemental Figure 2). Each IRF8 ChIP-seq peak contained composite elements for IRF and ETS. Importantly, this region was tightly associated with high levels of the H3K4me1 modification and PU.1 binding in IRF8-tranduced cells but not in empty MSCV-transduced cells. Because the next gene, a predicted pseudogene Gm12518, is located 855 kb away, Klf4 is the gene nearest to the above-mentioned IRF8 binding sites. In addition, there was no evidence of transcriptional changes in the next three genes including Gm12518, according to the ChIP-seq results for H3K4me3 and H3K36me3 (markers of initiation and elongation, respectively; data not shown) or by microarray. Therefore, we infer that the above-mentioned distal region is likely to function as a large cis-regulatory enhancer element for the Klf4 gene only when bound by IRF8.
IRF8 and PU.1 binding and the H3K4me1 enhancer signature at the Klf4 gene locus. (A) UCSC genome browser image of tag density plots for IRF8, PU.1, and H3K4me1 ChIP-seq and input DNA at the Klf4 gene locus in empty MSCV-transduced Tot2 cells or IRF8-transduced Tot2 cells. H3K4me1 ChIP-seq peaks identified by SICER and PU.1 and IRF8 ChIP-seq peaks by HOMER are indicated as bars above the density plots. The genomic sequences of each IRF8 ChIP-seq peak are shown (peaks A-F; see also supplemental Figure 2). (B) Direct activation of the Klf4 gene by IRF8. IRF8-estrogen receptor (ER) –transduced Tot2 cells were treated with β-estradiol (Est) (1 µM) and cycloheximide (CHX) (1 µg/mL). CHX was added 10 minutes before the addition of Est. Transcript levels of Klf4 and Myc were quantified in biological triplicates by qRT-PCR. Data were normalized by Actb levels and shown as values relative to those in ER alone–transduced Tot2 cells treated under the same conditions (mean ± standard error). (*) P < .05 (Student t test). NS, not significant.
IRF8 and PU.1 binding and the H3K4me1 enhancer signature at the Klf4 gene locus. (A) UCSC genome browser image of tag density plots for IRF8, PU.1, and H3K4me1 ChIP-seq and input DNA at the Klf4 gene locus in empty MSCV-transduced Tot2 cells or IRF8-transduced Tot2 cells. H3K4me1 ChIP-seq peaks identified by SICER and PU.1 and IRF8 ChIP-seq peaks by HOMER are indicated as bars above the density plots. The genomic sequences of each IRF8 ChIP-seq peak are shown (peaks A-F; see also supplemental Figure 2). (B) Direct activation of the Klf4 gene by IRF8. IRF8-estrogen receptor (ER) –transduced Tot2 cells were treated with β-estradiol (Est) (1 µM) and cycloheximide (CHX) (1 µg/mL). CHX was added 10 minutes before the addition of Est. Transcript levels of Klf4 and Myc were quantified in biological triplicates by qRT-PCR. Data were normalized by Actb levels and shown as values relative to those in ER alone–transduced Tot2 cells treated under the same conditions (mean ± standard error). (*) P < .05 (Student t test). NS, not significant.
To examine whether IRF8 directly regulates the expression of the Klf4 gene, we transduced an estrogen-inducible IRF8-estrogen receptor (ER) chimera into Tot2 cells and stimulated the cells with β-estradiol (Est) as previously described.20 IRF8-ER becomes active only upon Est stimulation. qPCR with reverse transcription (qRT-PCR) revealed that a one-hour Est treatment induced Klf4 messenger RNA (mRNA) expression in IRF8-ER–transduced cells (Figure 3B). This induction occurred even in the presence of cycloheximide, suggesting that IRF8-ER induces Klf4 without requiring de novo protein synthesis. In contrast, the suppression of an indirect target gene Myc was blocked when cycloheximide was added as previously reported.20 Taken together, these results suggest that Klf4 is a direct target gene of IRF8.
IRF8 is essential for monocyte differentiation in vivo
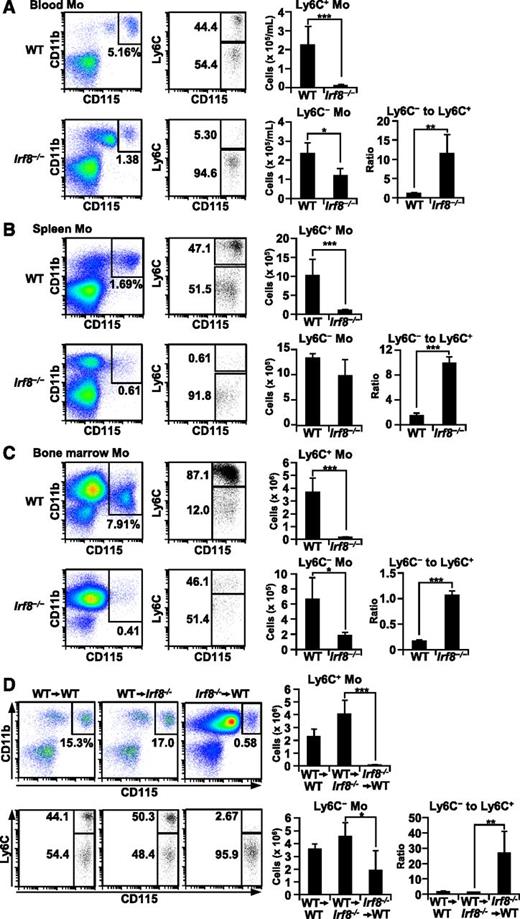
It has been reported that KLF4 is crucial for the development of Ly6C+ monocytes.12 Although IRF8 is important for macrophage development, little is known about its role in monocyte development in vivo. Given the prediction that KLF4 may be an important downstream transcription factor, we asked whether Irf8−/− mice have similar abnormalities to those seen in Klf4−/− chimeric mice. We found that blood Ly6C+ monocytes were severely diminished in Irf8−/− mice, and that Ly6C− monocyte counts were also reduced relative to levels in wild-type (WT) mice (Figure 4A). Accordingly, ratios of the numbers of Ly6C− monocytes to Ly6C+ monocytes in the blood of Irf8−/− mice were significantly higher than those in WT mice. Splenic Ly6C+ monocyte counts35 were also severely reduced (Figure 4B). Numbers of Ly6C− monocytes in the spleen tended to be lower in Irf8−/− mice than in WT mice, although the differences were not statistically significant. Bone marrow CD117− CD115+ monocytic cells in WT mice consist of approximately 90% Ly6C+ cells and 10% Ly6C− cells.6,36 The counts of both Ly6C+ and Ly6C− bone marrow monocytic cells were significantly decreased in Irf8−/− mice (Figure 4C). Thus, IRF8 is required for the development of both monocyte subsets, especially Ly6C+ monocytes. These abnormalities of monocyte development in Irf8−/− mice were similar to or more severe than those in previously reported Klf4−/− chimeric mice.
Irf8−/− mice lack Ly6C+ monocytes and have fewer Ly6C− monocytes than WT mice. (A-C) Monocytes (Mo) in the peripheral blood (A), spleen (B), and bone marrow (C) from WT or Irf8−/− mice. Monocytes were detected as CD11b+ CD115+ cells and then further analyzed for Ly6C expression. Splenic monocytes were first gated as side scatter (SSC)low cells. Bone marrow CD117+ cells were gated out to exclude progenitor cells. Absolute numbers (per 1 mL blood, per spleen, or per tibias plus femurs from a mouse) of Ly6C+ and Ly6C− monocytes are shown in the bar graphs. The ratios of Ly6C+ monocytes to Ly6C− monocytes are also shown. (D) Peripheral blood monocytes in mice that received bone marrow transplants. Irradiated WT recipients reconstituted using WT (WT→WT) or Irf8−/− (Irf8−/−→WT) bone marrow cells (1 × 107) or irradiated Irf8−/− recipients reconstituted using WT (WT→Irf8−/−) bone marrow cells (1 × 107) for 4 weeks were analyzed as in (A-C). All values are mean ± standard deviation from four mice of each genotype. (*) P < .05, (**) P < .01, and (***) P < .001 (Student t test).
Irf8−/− mice lack Ly6C+ monocytes and have fewer Ly6C− monocytes than WT mice. (A-C) Monocytes (Mo) in the peripheral blood (A), spleen (B), and bone marrow (C) from WT or Irf8−/− mice. Monocytes were detected as CD11b+ CD115+ cells and then further analyzed for Ly6C expression. Splenic monocytes were first gated as side scatter (SSC)low cells. Bone marrow CD117+ cells were gated out to exclude progenitor cells. Absolute numbers (per 1 mL blood, per spleen, or per tibias plus femurs from a mouse) of Ly6C+ and Ly6C− monocytes are shown in the bar graphs. The ratios of Ly6C+ monocytes to Ly6C− monocytes are also shown. (D) Peripheral blood monocytes in mice that received bone marrow transplants. Irradiated WT recipients reconstituted using WT (WT→WT) or Irf8−/− (Irf8−/−→WT) bone marrow cells (1 × 107) or irradiated Irf8−/− recipients reconstituted using WT (WT→Irf8−/−) bone marrow cells (1 × 107) for 4 weeks were analyzed as in (A-C). All values are mean ± standard deviation from four mice of each genotype. (*) P < .05, (**) P < .01, and (***) P < .001 (Student t test).
To examine whether the defect in monocyte differentiation in Irf8−/− mice was intrinsic to bone marrow–derived cells, we transplanted Irf8−/− or WT bone marrow cells into irradiated Irf8−/− or WT recipients. Engraftment of WT bone marrow in Irf8−/− recipients restored the population of both Ly6C+ and Ly6C− monocytes to normal levels. In contrast, after reconstitution of WT mice with Irf8−/− bone marrow cells, only very small numbers of Ly6C+ monocytes were detected, and Ly6C− monocyte counts were also reduced in the blood (Figure 4D). As a result, the ratio of Ly6C− monocyte counts to Ly6C+ monocyte counts in mice transplanted with Irf8−/− bone marrow cells was dramatically increased. These results suggest that the defective monocyte development seen in Irf8−/− mice is intrinsic to bone marrow-derived cells. As expected, neutrophilia was observed in both Irf8−/− mice and Irf8−/− bone marrow-transplanted WT mice.
Inflammatory macrophages are severely diminished in Irf8−/− mice
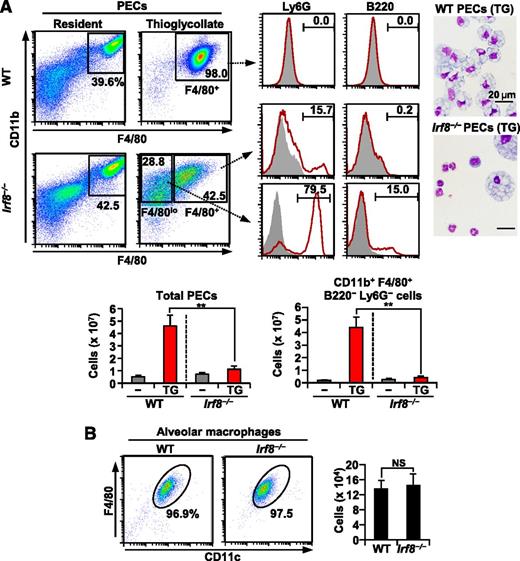
During peritonitis, Ly6C+ monocytes migrate into the peritoneal cavity and eventually differentiate into F4/80+ inflammatory macrophages.37,38 We tested whether IRF8 deficiency affects inflammatory macrophages in a sterile peritonitis model induced by injection of thioglycollate medium (TG). Before TG injection, the numbers of resident peritoneal CD11b+ F4/80hi macrophages were comparable between WT and Irf8−/− mice (Figure 5A). Four days after TG injection, a large number of inflammatory macrophages were harvested from WT mice. In Irf8−/− mice, however, TG injection failed to significantly increase the total number of peritoneal exudate cells (PECs). Furthermore, while 98% of WT PECs induced by TG were CD11b+ F4/80+ macrophages, only 42.5% of Irf8−/− PECs after TG injection were CD11b+ F4/80+. We confirmed that most of the CD11b+ F4/80lo/− cells in Irf8−/− mice are indeed nonmacrophage cells because 79.5% and 15.7% were Ly6G+ neutrophils and B220+ B cells, respectively. On the other hand, CD11b+ F4/80+ cells in Irf8−/− mice contained some Ly6G+ neutrophils (15.7%). We therefore calculated the numbers of CD11b+ F4/80+ B220− Ly6G− cells after TG injection as inflammatory macrophages, which were severely decreased in Irf8−/− mice. Wright-Giemsa stains of TG-induced PECs confirmed these findings. These results strongly support our finding that Ly6C+ monocytes are absent in Irf8−/− mice.
Inflammatory macrophages are severely diminished in Irf8−/− mice. (A) Peritoneal exudate cells (PECs) from WT and Irf8−/− mice 4 days after the intraperitoneal injection of TG. Representative data from flow cytometric analysis and Wright-Giemsa stains are shown in the upper panels. The absolute numbers of total PECs and CD11b+ F4/80+ B220− Ly6G− cells are shown in the bottom bar graphs. Values are mean ± standard deviation from three mice of each genotype. (B) Bronchoalveolar lavage cells. The absolute numbers of CD11c+ F4/80+ alveolar macrophages are shown in the bar graph. Values are mean ± standard deviation from four mice of each genotype. (**) P < .01 (Student t test). NS, not significant.
Inflammatory macrophages are severely diminished in Irf8−/− mice. (A) Peritoneal exudate cells (PECs) from WT and Irf8−/− mice 4 days after the intraperitoneal injection of TG. Representative data from flow cytometric analysis and Wright-Giemsa stains are shown in the upper panels. The absolute numbers of total PECs and CD11b+ F4/80+ B220− Ly6G− cells are shown in the bottom bar graphs. Values are mean ± standard deviation from three mice of each genotype. (B) Bronchoalveolar lavage cells. The absolute numbers of CD11c+ F4/80+ alveolar macrophages are shown in the bar graph. Values are mean ± standard deviation from four mice of each genotype. (**) P < .01 (Student t test). NS, not significant.
Given that Ly6C− monocytes have the potential to develop into alveolar macrophages,1,3 we next collected bronchoalveolar lavage cells and counted alveolar macrophages (Figure 5B). Irf8−/− mice had F4/80+ CD11c+ alveolar macrophage counts comparable to those in WT mice. Thus, although decreased in number, Ly6C− monocytes in Irf8−/− mice retain their ability to supply at least certain types of resident macrophages.
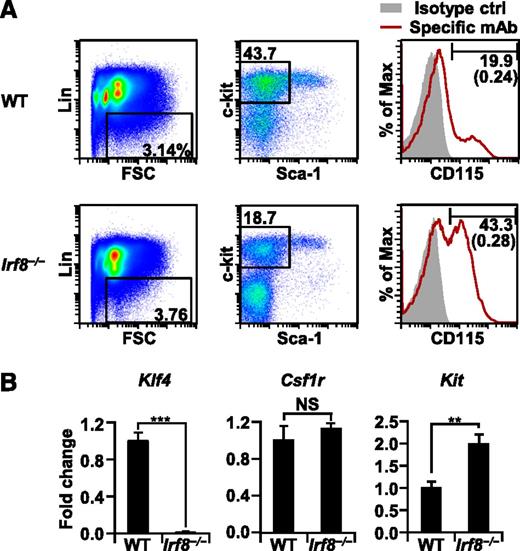
The expression of Klf4 is severely reduced in Irf8−/− MDPs
Previous studies have shown that the defects of monocytes in Klf4−/− chimeric mice are likely to result from the lack of KLF4 expression in their progenitor cells.12,39,40 Recent reports suggest that commitment to differentiation into monocytes/macrophages or DCs occurs at the stage of MDPs in vivo.2 Hence, we sorted Lin− CD115+ CD117int/+ MDPs6,7 from WT and Irf8−/− mice (Figure 6A) and analyzed their expression levels of Klf4 mRNA (Figure 6B). Strikingly, expression of Klf4 was lost in Irf8−/− MDPs. In contrast, the expression level of Csf1r in Irf8−/− MDPs is comparable to that in WT MDPs, and Kit expression was somewhat elevated in Irf8−/− MDPs. These data suggest that IRF8 is indispensable for the expression of Klf4 in MDPs.
Irf8−/− MDPs do not express Klf4 mRNA. (A) Flow cytometric analysis of Lin− Sca-1− CD117int/+ CD115+ MDPs. Numbers in parentheses indicate percentages of MDPs relative to whole bone marrow cells. Similar results were obtained in three other independent experiments. (B) Lin− Sca-1− CD117int/+ CD115+ MDPs were isolated from WT and Irf8−/− mice by cell sorting. Klf4, Csf1r, and Kit mRNA expression levels were analyzed by qRT-PCR in triplicate using the ΔΔCT method and were normalized by comparison with levels of Gapdh (mean ± standard deviation). (**) P < .01, (***) P < .001 (Student t test).
Irf8−/− MDPs do not express Klf4 mRNA. (A) Flow cytometric analysis of Lin− Sca-1− CD117int/+ CD115+ MDPs. Numbers in parentheses indicate percentages of MDPs relative to whole bone marrow cells. Similar results were obtained in three other independent experiments. (B) Lin− Sca-1− CD117int/+ CD115+ MDPs were isolated from WT and Irf8−/− mice by cell sorting. Klf4, Csf1r, and Kit mRNA expression levels were analyzed by qRT-PCR in triplicate using the ΔΔCT method and were normalized by comparison with levels of Gapdh (mean ± standard deviation). (**) P < .01, (***) P < .001 (Student t test).
KLF4 acts downstream of IRF8 to induce monocyte/macrophage differentiation
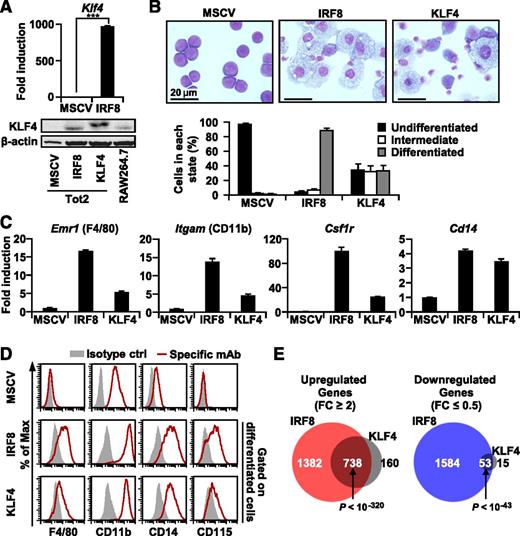
To examine whether and to what extent KLF4 plays a biological role downstream of IRF8, we transduced IRF8 or KLF4 into Irf8−/− Tot2 cells using an MSCV retrovirus. IRF8 induced KLF4 expression at the mRNA and protein levels (Figure 7A). As expected, more than 90% of the IRF8-transduced cells differentiated into monocytes/macrophages on day 4 (Figure 7B). Importantly, KLF4 transduction resulted in monocyte/macrophage differentiation in the absence of IRF8 although not as strongly as IRF8. One third of the KLF4-transduced cells exhibited morphologic changes typical of monocytes/macrophages, such as enlargement of the cytoplasm filled with vacuoles and condensation of nuclei, and adhered strongly to culture dishes. Half the remaining KLF4-transduced cells exhibited partial morphologic changes but did not adhere to culture dishes. The observation that KLF4-transduced monocytes that adhered to culture dishes expressed a higher level of Klf4 mRNA than floating cells (supplemental Figure 7A) suggests that the phenotypical heterogeneity among KLF4-transduced cells may arise from graded expression levels of ectopic KLF4 expression, which is common for retrovirally expressed proteins. Tot2 cells transduced with KLF4 expressed monocyte markers including F4/80, CD11b, CD14, and CD115 at both mRNA and surface protein levels (Figure 7C-D and supplemental Figure 7B). Nonetheless, their levels of expression were lower than those in IRF8-transduced cells. This was the case even when morphologically well-differentiated cells were analyzed (Figure 7D, gated by forward and side scatters; see supplemental Figure 7B for time course data on a whole population of transduced cells). Of note, KLF4 induced cell cycle arrest as strongly as IRF8 (supplemental Figure 8A). To examine the function of KLF4-transduced cells, we performed a phagocytosis assay using Escherichia coli bioparticles labeled with fluorescein isothiocyanate. The observation that KLF4-transduced, differentiated cells showed a level of phagocytic activity comparable to IRF8-transduced cells suggests that they are bona fide phagocytes (supplemental Figure 8B). Taken together, KLF4 can drive Irf8−/− myeloid progenitor cells to differentiate into growth-arrested monocytes/macrophages, although the effect of KLF4 appears to be smaller than that of IRF8.
The IRF8-KLF4 axis regulates the development of monocytes. (A) Induction of KLF4 expression by IRF8. Tot2 cells transduced with empty MSCV-puro, MSCV-IRF8-puro, or MSCV-KLF4-FLAG-puro were analyzed by qRT-PCR in triplicate (mean ± standard deviation) and western blotting analysis on day 4. RAW264.7 cells were also loaded for comparison. Data are representative of three independent experiments with similar results. (***) P < .001 (Student t test). (B) Wright-Giemsa stains of Tot2 cells transduced with indicated MSCV. Cells were classified into three categories (differentiated, intermediate, and undifferentiated) on day 4 (mean ± standard deviation). (C) Induction of monocyte-related genes. Transcript levels were analyzed by qRT-PCR on day 4 as in (A). (D) Surface marker analysis. Cells on day 4 were stained with the indicated antibodies or isotype-matched control antibodies and analyzed by flow cytometry. Forward scatter (FSC)hi SSChi cells were gated to analyze differentiated cells. Data are representative of three independent experiments. (E) Venn diagram for the relationship between genes that displayed more than a 2-fold change in expression 2 days after transduction with IRF8 or KLF4. Gene expression profiling by microarray was performed in biological duplicates as in Figure 1. P values by Fisher’s exact test are indicated.
The IRF8-KLF4 axis regulates the development of monocytes. (A) Induction of KLF4 expression by IRF8. Tot2 cells transduced with empty MSCV-puro, MSCV-IRF8-puro, or MSCV-KLF4-FLAG-puro were analyzed by qRT-PCR in triplicate (mean ± standard deviation) and western blotting analysis on day 4. RAW264.7 cells were also loaded for comparison. Data are representative of three independent experiments with similar results. (***) P < .001 (Student t test). (B) Wright-Giemsa stains of Tot2 cells transduced with indicated MSCV. Cells were classified into three categories (differentiated, intermediate, and undifferentiated) on day 4 (mean ± standard deviation). (C) Induction of monocyte-related genes. Transcript levels were analyzed by qRT-PCR on day 4 as in (A). (D) Surface marker analysis. Cells on day 4 were stained with the indicated antibodies or isotype-matched control antibodies and analyzed by flow cytometry. Forward scatter (FSC)hi SSChi cells were gated to analyze differentiated cells. Data are representative of three independent experiments. (E) Venn diagram for the relationship between genes that displayed more than a 2-fold change in expression 2 days after transduction with IRF8 or KLF4. Gene expression profiling by microarray was performed in biological duplicates as in Figure 1. P values by Fisher’s exact test are indicated.
Characterization of genes regulated by IRF8 and/or KLF4
Transcriptome analysis identified 898 upregulated genes and 68 downregulated genes in KLF4-transduced cells (Figure 7E, supplemental Figure 8C, and supplemental Tables 7-8). We found that 82.2% of the genes upregulated by KLF4 and 77.9% of the genes downregulated by KLF4 were also regulated by IRF8. Conversely, only 34.8% of genes upregulated by IRF8 and 3.2% of genes downregulated by IRF8 were also regulated by KLF4. These results indicate that KLF4 is responsible for inducing a fraction of IRF8 target genes. Of note, among 1045 genes indirectly induced by IRF8, 378 genes (36.2%) were upregulated by KLF4.
GO analysis showed that many of the genes upregulated by KLF4 had annotations related to “immunity,” as was found for genes upregulated by IRF8 (supplemental Figure 8D and supplemental Table 9). However, more detailed GO analysis revealed that a subset of immunity-related annotations was missing in KLF4-regulated genes when compared with IRF8-regulated genes, especially “antigen presentation”-related annotations (supplemental Table 10). Conversely, the genes commonly induced by IRF8 and KLF4 preferentially had annotations related to “chemotaxis” and “locomotion” when compared with those induced only by IRF8 (supplemental Table 11).
Discussion
We used genome-wide approaches to show that IRF8 binds to promoter-proximal and promoter-distal sites and induces gene expression to drive myeloid progenitors toward differentiation into monocytes/macrophages. In most cases, IRF8 colocalizes with the transcription factor PU.1. At the distal sites, binding of IRF8 leads to increased formation of H3K4me1, a hallmark of enhancers. The Klf4 gene, which encodes a transcription factor essential for the development of a subset of monocytes, is directly induced by IRF8, presumably via such distal binding. Indeed, the KLF4 binding motif is significantly enriched at both promoter and enhancer regions of the genes that are induced but not bound by IRF8. Moreover, the introduction of KLF4 into Irf8−/− myeloid progenitor cells causes monocyte/macrophage differentiation in the absence of IRF8. Two pieces of in vivo evidence underscore the importance of the IRF8-KLF4 axis in monocyte differentiation. First, Irf8−/− MDPs, the progenitor population of monocytes, fail to express Klf4. Second, Irf8−/− mice display similar but more severe abnormalities in monocyte development than those seen in Klf4−/− chimeric mice. On the basis of these results, we conclude that the IRF8-KLF4 transcription factor cascade is essential for monocyte development.
Although IRF8 was originally identified as a transcriptional repressor, this study convincingly demonstrates that IRF8 binding is associated mainly with upregulation of gene expression during monocyte/macrophage development. Of importance in this regard is the finding that distal binding of IRF8 potently induces H3K4me1 with a bimodal distribution, which is a hallmark of enhancer elements. Given the recent insight that enhancers are specific to cell types and strongly correlate to lineage-specific gene expression on a global scale,31 it is likely that the formation of enhancers by IRF8 is key to the commitment of myeloid progenitors to the monocyte lineage. It has been suggested that PU.1 induces chromatin remodeling and the local deposition of H3K4me1 at its binding sites.33,34 The motifs most commonly found in distal IRF8 binding peaks were EICE and IECS, and IRF8 indeed colocalized with PU.1. Thus, it is highly likely that IRF8 cooperates with PU.1 to drive the formation of H3K4me1. It will be important to investigate detailed mechanisms by which these transcription factors regulate chromatin.
We noted, however, that approximately half of IRF8-induced genes are not bound by IRF8. This indicates the possibility that these genes may be indirectly regulated by IRF8 via downstream transcription factors. DNA motif analysis of the cis-regulatory regions of these genes predicted the IRF8-KLF4 axis, and its biological significance was demonstrated by subsequent biological analyses. IRF8 directly binds to multiple sites within the long (120 kb) distal region located 208 kb upstream of the Klf4 TSS, which is then marked with the H3K4me1 enhancer chromatin signature. Given that an enhancer 1 Mb from the KLF4 TSS has been reported to be functional,41 it is conceivable that IRF8 promotes Klf4 expression by shaping the distal large enhancer identified in this study during monocyte differentiation. In addition, our finding that β-estradiol–activated IRF8-ER rapidly induces Klf4 mRNA in the presence of cycloheximide indicates that Klf4 is a direct IRF8 target. The definitive in vivo finding indicating the importance of the IRF8 in Klf4 expression is that Irf8−/− MDPs fail to express Klf4 mRNA.
Gene expression profiling clearly shows that KLF4 mediates upregulation of a subset of IRF8 target genes. Moreover, KLF4 indeed causes partial monocyte/macrophage differentiation even in the absence of IRF8. GO analysis delineated the range of IRF8 target genes controlled by mechanisms dependent on and independent of KLF4. Although both are related to immunity, the KLF4-dependent IRF8 target genes are preferentially associated with annotations related to chemotaxis and locomotion, especially chemokines (CCL2, CCL3, CCL4, CCL5, and CCL6) known to be produced primarily by monocytes. In contrast, the KLF4-independent IRF8 target genes show significant association with annotations related to antigen presentation. Indeed, IRF8 directly binds to and upregulates multiple genes encoding major histocompatibility complex class II molecules (H2-Aa, H2-DMb2, H2-K1, and H2-Q7) and class II invariant chain (CD74), some of which have been reported previously to be regulated by IRF8.30
It is possible that transcriptional regulators other than KLF4 also play roles downstream of IRF8. For example, IRF8-induced transcription factor genes such as Egr1, Egr2, Maf, and MafB have also been implicated in monocyte differentiation.13-15,42 The binding motifs of the transcription factors encoded by them are significantly enriched in the cis-regulatory regions of genes targeted indirectly by IRF8. All of these genes, except Maf, are bound by IRF8. DNA motif analysis indicated Bcl6, Lhx5, Lhx2, and Ahrr as additional candidates, although Lhx5 and Ahrr are indirect IRF8 targets. Interestingly, some of these candidates, such as Lhx5 and Bcl6, are also induced by KLF4 (13.7-fold and 17.3-fold, respectively). The induction of the indirect IRF8 target gene Lhx5 by KLF4 suggests the presence of multistep transcription factor cascades. The induction of IRF8-bound Bcl6 by KLF4 may represent simultaneous regulation by IRF8 and KLF4, which may explain why KLF4 alone is weaker than IRF8 in inducing several common downstream genes including Bcl6, Emr1, Itgam, and Csf1r. Indeed, among the 738 genes commonly induced by IRF8 and KLF4, 111 genes, including Bcl6, have both IRF8 ChIP peak(s) and KLF4 binding motif(s). It is also possible that IRF8 and KLF4 co-regulate genes indirectly (ie, the commonly induced genes could be bound by transcription factors downstream of IRF8 other than KLF4). In terms of the regulation of immune responses of monocytes/macrophages, it will be important to examine the roles of Atf3, Atf7, and Irf5 as IRF8 targets.
Our results also have implications for elucidating the pathways that define the development of distinct subsets of monocytes. Whereas Irf8−/− mice almost lost the ability to produce Ly6C+ monocytes, they still retained the ability to generate Ly6C− monocytes, albeit with reduced efficiency compared with WT mice. Previous reports have suggested that Ly6C− monocytes arise from Ly6C+ monocytes.8,43 However, the fact that Ly6C− monocytes exist despite the absence of Ly6C+ monocytes in Irf8−/− mice indicates the presence of another pathway, presumably originating with MDPs, which results in the generation of Ly6C− monocytes. It was recently reported that both Ly6C+ and Ly6C− monocyte counts were reduced to the same extent in apolipoprotein E (Apo-E) –deficient mice transplanted with Irf8−/− bone marrow cells compared with those transplanted with WT bone marrow cells.44 The authors further reported that the number of TG-elicited Irf8−/− peritoneal macrophages was comparable to that in WT mice. The possible reasons for the discrepancy between our results and theirs may include their use of ApoE-deficient recipient mice given a high-fat diet (which influences monocyte homeostasis45 ), their monocyte detection method (which did not use CD115), and the low efficiency of macrophage recruitment following TG injection using their experimental conditions.
In conclusion, our study has unveiled genome-wide behaviors of IRF8 and has shown that the IRF8-KLF4 transcription factor cascade plays a critical role in the differentiation of MDPs to Ly6C+ monocytes. Taken together with the essential role of IRF8 in the development of several DC subsets, our findings further implicate the fundamental role of IRF8 in the mononuclear phagocyte system.
ChIP-seq and microarray data reported in this article have been deposited in the Gene Expression Omnibus database (GSE38825).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Alexei A. Sharov at National Institutes of Health and Dr Akinori Sarai at Kyushu Institute of Technology for their valuable advice on statistics, and Mr Masahiro Yoshinari at Yokohama City University for his help with experiments.
This work was supported by KAKENHI grants-in-aid 24390246, 23659492, and 24118002 (T.T.), 24790322 (D.K.), and 23590343 (A.N.) from the Japan Society for the Promotion of Science, a Special Coordination Fund for Promoting Science and Technology “Creation of Innovation Centers for Advanced Interdisciplinary Research Areas” from MEXT (T.T.), a grant for Strategic Research Promotion from Yokohama City University (T.T.), and a grant from the Yokohama Foundation for Advancement of Medical Science (T.T.). The supercomputing resource was provided by Human Genome Center of the Institute of Medical Science at the University of Tokyo.
Authorship
Contribution: D.K. and T.T. designed the research. D.K., A.N., M.Y., H.S., and M.U. performed experiments. D.K., N.O., A.N., J.N., T.B., H.S., and T.T. analyzed data. D.K., N.O., K.O., and T.T. wrote the manuscript. N. Miyake and N. Matsumoto guided next-generation sequencing. M.N. performed critical animal maintenance. K.O. provided critical materials. T.T. supervised the project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for N.O. is Genome Science Division, Research Center for Advanced Science and Technology, The University of Tokyo, Tokyo, Japan.
Correspondence: Tomohiko Tamura, Department of Immunology, Yokohama City University Graduate School of Medicine, 3-9 Fukuura, Kanazawa-ku, Yokohama 236-0004, Japan; e-mail: tamurat@yokohama-cu.ac.jp.
References
Author notes
D.K., N.O., A.N., and M.Y. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal