In this issue of Blood, Heideman and colleagues show that the major class I histone deacetylases (HDACs) HDAC1 and HDAC2 can act to suppress tumors in mouse thymocytes.
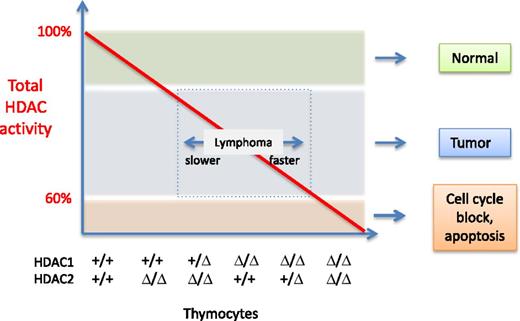
Total HDAC activity (red line) present in thymocytes as a function of HDAC1 and HDAC2. The genotype of the different samples is indicated on the x-axis: +, wild-type allele; Δ, deleted allele. For simplicity, the deacetylase activity function is drawn as a straight line. However, the authors determined that HDAC1 contributes to more activity than HDAC2. The outcomes observed are indicated on the right of the graph, and the speed at which lymphomagenesis develops is depicted by the double arrow in the box. Illustration by Patrick Matthias.
Total HDAC activity (red line) present in thymocytes as a function of HDAC1 and HDAC2. The genotype of the different samples is indicated on the x-axis: +, wild-type allele; Δ, deleted allele. For simplicity, the deacetylase activity function is drawn as a straight line. However, the authors determined that HDAC1 contributes to more activity than HDAC2. The outcomes observed are indicated on the right of the graph, and the speed at which lymphomagenesis develops is depicted by the double arrow in the box. Illustration by Patrick Matthias.
Acetylation of proteins on lysine residues has been recognized as a crucial posttranslational modification that is now second only to phosphorylation in its prevalence.1 Histone deacetylases are a family of enzymes that remove acetyl groups from histone N-terminal tails, thereby contributing to chromatin condensation and the modulation of gene expression and of other chromatin-based processes.2 In addition, HDACs also can deacetylate an increasing number of nonhistone proteins, impinging on diverse cellular processes.
Inhibitors of HDACs, such as trichostatin A, were identified more than 20 years ago and were rapidly shown to have remarkable biological properties, such as induction of differentiation in cellular systems and a marked antiproliferative potential when applied to transformed cells in culture.3 These early observations sparked an enormous interest in HDAC inhibition as a novel therapeutic modality; today 2 pan-inhibitors are approved for treatment of cutaneous T-cell lymphoma (CTCL). HDAC inhibition represents the first available epigenetic therapy and it is currently considered for a variety of other cancers in addition to CTCL, as well as for neurodegeneration and autoimmunity. However, it has not yet been established which HDAC(s) have to be inhibited under which condition. It is generally assumed that specific inhibitors might have fewer side effects, although their clinical efficacy remains to be demonstrated.
Heideman et al4 generated genetically modified mice lacking HDAC1 and HDAC2 in thymocytes. By making a systematic combination of alleles, they created a series of mice expressing a gradient of deacetylase activity as a function of HDAC1/HDAC2 levels. Unexpectedly, they found that in mice lacking HDAC1 and having a single HDAC2 allele, immature thymocytes accumulated in great numbers, and monoclonal lymphoblastic lymphomas were observed, which led to death of the animals at age 5 to 15 weeks. A similar but slower-appearing phenotype was observed in mice of other genotypes: in the absence of HDAC1, most mice developed lymphomas between ages 15 and 25 weeks, and mice having 1 copy of HDAC1 but no HDAC2 developed lymphoma between ages 18 and 28 weeks. In contrast, when both enzymes were fully ablated, lymphomagenesis was abrogated, owing to a block in early thymocyte development. Using thymocytes of the different genotypes, the authors showed that the various HDAC1/HDAC2 combinations result in different levels of overall deacetylase activity, with HDAC1 contributing to more activity than HDAC2. Based on the remarkable correlation between the time of lymphoma development and the level of overall HDAC activity detected in the thymocytes, the authors postulate that in this system, lymphomagenesis is the outcome of an insufficient level of HDAC activity, elicited by deletion of either HDAC1 or HDAC2 (see Figure).
The authors go on to show that in the T-cell lymphomas, the p53 pathway is functionally inactivated, although p53 does not appear to be mutated. They demonstrate that the Myc gene is overexpressed in the lymphomas due to chromosomal amplification. Furthermore, they show that Myc-collaborating genes, such as the p53 suppressor Jdp2, are overexpressed in an HDAC1/HDAC2–dependent manner. Finally, the authors provide evidence that Jdp2 overexpression is critical for the survival of these lymphoma cells.
Beyond these novel mechanistic insights, which are very relevant, the main merit of this important study—and of another recently published study with similar conclusions5 —is to clearly demonstrate that, at least in thymocytes, HDAC1 and HDAC2 can act as tumor suppressors. The logical interpretation put forward is that the level of deacetylase activity, controlled here by HDAC1 and HDAC2, is what matters for the tumor suppression function. However, it is challenging to unravel what is due to a general reduction of HDAC activity vs specific effects via HDAC1 and/or HDAC2. These observations are in some contrast to the general concept of HDAC function, which posits that cancer cells (often) have elevated HDAC activity that helps them to proliferate (eg, by directly repressing some of the small cyclin-dependent kinase inhibitor genes, such as p21 or p576,7 ). In this classical scenario, HDAC inhibition counteracts uncontrolled cellular proliferation and is therefore beneficial.
A previous unrelated study showed that liver-specific ablation of HDAC3, another class I HDAC, resulted in hepatocellular carcinomas.8 This observation was linked to increased DNA damage and loss of genomic stability in hepatocytes. Here, too, a possible interpretation could be that insufficient overall deacetylase activity was critical. Still, one could also imagine that biochemical functions other than catalytic activity could be influenced by the reduced levels of HDAC1, HDAC2, or HDAC3. Furthermore, are the tumor-suppression effects a specific property of only HDAC1, HDAC2, and HDAC3, or could the same tumorigenic outcomes be achieved with other combinations of HDAC ablation (inhibition), perhaps also involving class II HDACs?
The answer is not known yet, but the clear message of the findings by Heideman et al is that partial HDAC inhibition, which may be the norm rather than the exception in therapeutic settings, may in itself bring undesired and previously unforeseen effects. It will be interesting to see whether these unexpected effects are also observed in other cellular systems and whether inhibitor treatment at suboptimal doses may also lead to tumor development in model systems.
Conflict-of-interest disclosure: The author declares no competing financial interests.