Abstract
Hemophilia is a bleeding disorder that afflicts about 1 in 5000 males. Treatment relies upon replacement of the deficient factor, and response to treatment both in clinical research and practice is based upon subjective parameters such as pain and joint mobility. Existing laboratory assays quantify the amount of factor in plasma, which is useful diagnostically and prognostically. However, these assays are limited in their ability to fully evaluate the patient’s clot-forming capability. Newer assays, known as global assays, provide a far more detailed view of thrombin generation and clot formation and have been studied in hemophilia for about 10 years. They have the potential to offer a more objective measure of both the hemophilic phenotype as well as the response to treatment. In particular, in patients who develop inhibitors to deficient clotting factors and in whom bypassing agents are required for hemostasis, these assays offer the opportunity to determine the laboratory response to these interventions where traditional coagulation assays cannot. In this article we review the existing literature and discuss several controversial issues surrounding the assays. Last, a vision of future clinical uses of these assays is briefly described.
Background
An important principle in the medical management of patients and in clinical research is the ability to assess the outcomes of an intervention. Some simple yet vitally important examples include the management of patients with hypertension and hypercholesterolemia in whom the outcomes of a medical intervention—be it diet, exercise, or medications—can be determined easily by measuring blood pressure or assessing serum levels of the various cholesterol lipoproteins. Unfortunately, such simple and useful tests are not available for the management of patients with hemophilia who have inhibitors; for patients without inhibitors, measurement of factor levels provides useful information, though it could be argued that better assays are needed (see below).
Considering the advances that have been made in the treatment of hemophilia, it is surprising that essentially the entirety of disease management and clinical trial outcomes, including the regulatory approval of new agents, is based on highly subjective, nonvalidated, patient-reported outcome measures.1 The field of hemophilia sorely needs to develop objective endpoints that predict a clinical response. The most objective endpoint would be a device that could directly interrogate the area of bleeding (such as within a joint space) to demonstrate bleeding cessation. For the foreseeable future, however, such a device is unlikely to be available. Thus, the next option is to develop a test, such as a laboratory assay, that can predict the outcome of an intervention used to manage a bleed or to prevent bleeding during surgery. It should be pointed out that although existing factor assays offer some measure of prediction of clinical outcomes, they do not predict the hemostatic effect of bypassing agents, nor do they allow any direct comparison of the hemostatic effect of factor VIII (FVIII) or factor IX (FIX) replacement with the bypassing agents. Furthermore, factor assays do not provide a complete picture of the clotting process. For example, most clinical assays use fibrin formation as a means to assess hemostasis (prothrombin time [PT]2 and activated partial thromboplastin time [aPTT]2 ). In vitro, the formation of a visible fibrin clot occurs during the initiation phase of coagulation at very low levels of thrombin, when only 3% to 5% of the total amount of thrombin has been produced.3 The majority of thrombin (∼95%) is thus generated after clot formation when a great deal of hemostatic physiology is occurring, and it is not captured by the fibrin-clotting endpoints used commonly to evaluate the hemostatic process. Hemostasis does not appear to be synonymous with the endpoint of the fibrin-clotting reaction, and the latter is not a sufficient descriptor of the pathology associated with errors in the hemostatic process. As such, the hemostatic process cannot be adequately evaluated by a simple clotting endpoint assay.
It is now well established that clinical heterogeneity exists among patients with hemophilia who have the same degree of factor deficiency. In a longitudinal study, Aledort et al showed that 10% of severe hemophiliacs had a mild phenotype and exhibited no joint deterioration.4 This observation suggests that clotting factor assays do not provide the reliable clinical utility that cholesterol or glycosylated hemoglobin levels provide. Herein, we describe the current state of the art for global hemostatic assays as they pertain to the management of hemophilia patients and we provide a perspective on the future of this field.
Existing global assays used in hemophilia
Thus far, 2 types of assay have been studied in some detail over the past decade. One is based on the generation of thrombin, hereafter referred to as thrombin generation tests, and the other is based on measuring the viscoelastic properties of whole blood, hereafter referred to as viscoelastic tests. The thrombin generation tests use (platelet-poor or platelet-rich) plasma samples to determine the rate and extent of thrombin formed after a tissue factor stimulus. Thus, they indirectly assess clot formation by using thrombin generation as a surrogate. The viscoelastic tests measure the changes in elastic properties of whole blood during the process of clot formation and fibrinolytic dissolution.
Thrombin generation tests
Calibrated, automated thrombin generation
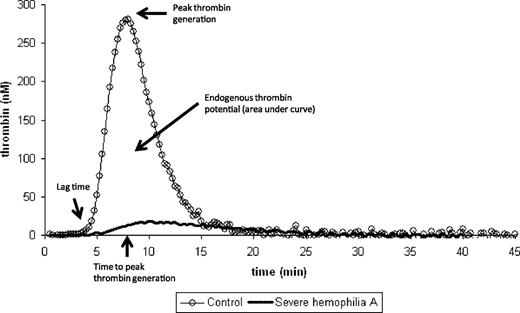
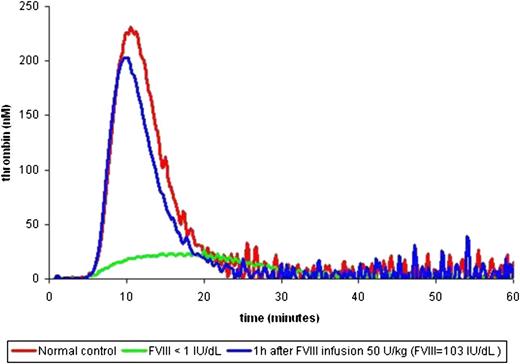
Hemker et al pioneered the conceptual methodology of the calibrated, automated thrombin generation method.5 The aim was to develop a simple method for quantifying continuous and dynamic properties of thrombin generation in the presence of fibrin(ogen) following a given tissue factor stimulus. The classic thrombin generation curve has a waveform from which a series of specified parameters can be calculated (lag time, thrombin generation velocity, peak thrombin concentration, time-to-peak thrombin, and endogenous thrombin potential; Figure 1).6 In principle, an abnormal thrombin generation curve can be characterized by the following: (1) a prolonged or shortened lag time, (2) reduced or increased peak thrombin, and/or (3) reduced or elevated endogenous thrombin potential (ETP).6 Figure 2 shows a thrombin generation curve of a severe hemophilia patient before and after a dose of FVIII concentrate, demonstrating dramatic changes in each of these 3 parameters.
Normal thrombin generation curve. The major parameters (lag time, time to peak thrombin generation, peak thrombin generation, and endogenous thrombin potential) of the thrombin generation curve are shown. For reference, a patient with severe hemophilia whereby all 3 parameters are severely affected is included.
Normal thrombin generation curve. The major parameters (lag time, time to peak thrombin generation, peak thrombin generation, and endogenous thrombin potential) of the thrombin generation curve are shown. For reference, a patient with severe hemophilia whereby all 3 parameters are severely affected is included.
Thrombin generation curve before and after treatment. Three thrombin generation curves are shown: normal (red), severe FVIII deficiency at baseline (green), and severe FVIII deficiency after an infusion of FVIII 50 IU/kg leading to a FVIII level of 103% (blue).
Thrombin generation curve before and after treatment. Three thrombin generation curves are shown: normal (red), severe FVIII deficiency at baseline (green), and severe FVIII deficiency after an infusion of FVIII 50 IU/kg leading to a FVIII level of 103% (blue).
Correlation of thrombin generation with clinical phenotype
Thrombin generation tests have been used for laboratory phenotyping of a variety of bleeding disorders. The best-characterized hemostatic dysfunction described by thrombin generation profiles is hemophilia A. In contrast to the categorical distinction between mild, moderate, and severe hemophilia based on assessment of functional levels of FVIII or FIX, the rate-specific characteristics of thrombin generation have been reported to illustrate and reflect the clinical heterogeneity of hemophilia.7-10 In particular, it has been reported that thrombin generation has been used to distinguish milder phenotypes of severe hemophilia despite similar low levels of FVIII (<1% of normal).8
Prediction of response to bypassing agents using thrombin generation
Thrombin generation measurements have been used to monitor substitution with bypassing agents such as recombinant factor VIIa (rFVIIa; Novoseven, Novo Nordisk, Bagsvaerd, Denmark) or plasma-derived activated prothrombin complex concentrates (pd-APCC; FEIBA, Baxter, Vienna, Austria).11-14 The overall experience with thrombin generation as a surrogate measure of hemostatic efficacy is still rather limited; however, the preliminary results appear promising. Prospective assessment of thrombin generation for dose prediction and monitoring of bypassing therapy in a case series of hemophilia patients with inhibitors undergoing elective surgery has recently been published.12 In this study, bypassing agent dosing was tailored by using a standardized 3-step protocol, as follows: (1) in vitro spiking experiments were used to evaluate thrombin generation with the addition of increasing concentrations of rFVIIa (0, 90, 180, 200, 240, 270 μg/kg) and FEIBA (0, 75, 100 U/kg) in order to determine the minimal dose of each agent that could normalize thrombin generation capacity; (2) ex vivo confirmation step wherein the more effective bypassing agent from the in vitro experiments was administered to the patient and thrombin generation was determined; and (3) perioperative treatment with the chosen bypassing agent at the specific dose that normalized the ETP and monitoring of bleeding during and after surgery. Because rFVIIa predominantly leads to FXa and FIXa generation on activated platelets, the hemostatic efficacy of rFVIIa was evaluated using platelet rich plasma (PRP). Because FEIBA is believed to have a different hemostatic mechanism, it was evaluated using platelet poor plasma (PPP). Preliminary results showed a good correlation between in vivo clinical response to bypassing agents and thrombin-generating capacity. Furthermore, the data suggested that thrombin generation may represent a surrogate marker for monitoring bypassing therapies in surgical situations.
Viscoelastic assays (thromboelastography/thromboelastometry)
Technical aspects
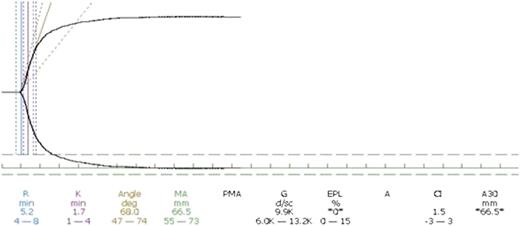
Hartert15 introduced the thrombelastographic principle, and it has been adopted in the computerized version of the TEG apparatus (Haemonetics, Braintree, MA). In 1996 Calatzis et al16 invented another component of thrombelastography, today called thromboelastometry (ROTEM, Pentapharm, Basel, Switzerland) in which the pin oscillates instead of the cup. Both TEG and ROTEM provide a digital signal that allows for additional computation of the continuous coagulation signal, leading to the derivation of several quantifiable parameters. Figure 3 demonstrates a normal TEG curve with its parameters. Both TEG and ROTEM provide a series of quantitative parameters that provide information on time to clot formation and propagation as well as information regarding clot firmness and elasticity.6 Importantly, both assays can characterize the later fibrinolytic phase, thereby providing unique information on clot stability.
Normal thromboelastography curve. The normal thromboelastographic curve is shown along with the resultant parameters along the bottom of the figure. R represents the time to clot initiation and is measured at the point that the 2 lines have separated 2 mm. K represents the clot propagation and is measured as the time from R until the curves are 20 mm apart. The angle represents the tangential angle of the curve with the horizontal and also represents clot propagation. MA is the maximal amplitude represented as the distance in millimeters between the 2 curves once they are parallel and represents peak clot rigidity.
Normal thromboelastography curve. The normal thromboelastographic curve is shown along with the resultant parameters along the bottom of the figure. R represents the time to clot initiation and is measured at the point that the 2 lines have separated 2 mm. K represents the clot propagation and is measured as the time from R until the curves are 20 mm apart. The angle represents the tangential angle of the curve with the horizontal and also represents clot propagation. MA is the maximal amplitude represented as the distance in millimeters between the 2 curves once they are parallel and represents peak clot rigidity.
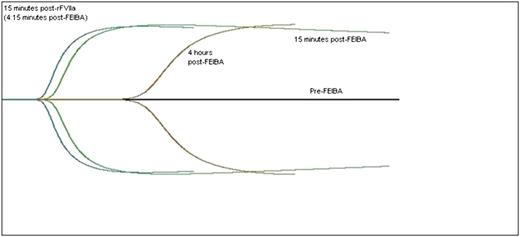
Thromboelastograph curve before and after treatment. The black horizontal line is the patient’s baseline, which is flat and demonstrates no clot formation. The green curve represents 15 minutes after a dose of the bypassing agent, FEIBA 75 IU/kg. The brown curve represents 4 hours after the FEIBA has been infused, demonstrating a degrading of the blood’s clot-forming ability. The blue curve represents 15 minutes after a dose of rFVIIa, 90 μg/kg, demonstrating the additive effect of the 2 agents.
Thromboelastograph curve before and after treatment. The black horizontal line is the patient’s baseline, which is flat and demonstrates no clot formation. The green curve represents 15 minutes after a dose of the bypassing agent, FEIBA 75 IU/kg. The brown curve represents 4 hours after the FEIBA has been infused, demonstrating a degrading of the blood’s clot-forming ability. The blue curve represents 15 minutes after a dose of rFVIIa, 90 μg/kg, demonstrating the additive effect of the 2 agents.
Methodology
Historically, the TEG assay used contact pathway activation; intrinsic activation remains the only licensed method for this assay when it is used in the management of surgical hemostasis.17 When the assay was adapted for use in hemophilia, both an intrinsic factor approach incorporating kaolin and a tissue factor approach (most often using Innovin, Siemens, Marburg, Germany) were used. In the early studies by Sørensen et al,18,19 a dilution of 1:17 000—corresponding to a tissue factor concentration approximating 0.35pM—was used, while in a study by Young et al,20 kaolin was used. Recently, it has become clear that when employing low concentrations of tissue factor, the intrinsic system must be inhibited with the addition of corn trypsin inhibitor (CTI).21 The principles of the tissue factor–initiated method include the addition of tissue factor to citrated whole blood containing CTI that is then placed in the cup and recalcified. The concentration and source of tissue factor can be varied, thereby offering the ability to modify the analytic conditions.18 The kaolin method is simpler because there is no need to prepare dilutions of tissue factor; however, it cannot be modified because the kaolin vials are premixed. Essentially, 1 mL of a whole blood sample is added to the kaolin vial and the vial is then gently inverted 3 to 4 times. The blood sample is then added to the cup and the sample is recalcified.
Regardless of the choice of activator, the TEG/ROTEM assay is adaptable to in vitro experiments by spiking samples with various products (eg, factor concentrates, antifibrinolytic agents, anticoagulants) according to the aims of the experiment. In addition, because TEG/ROTEM can evaluate fibrinolysis, the methods can be modified with the addition of tissue plasminogen activator to induce fibrinolysis and measure clot stability.22 Adapting the assays as such can demonstrate the presence of a fibrinolytic defect in hemophilia patients treated with bypassing agents and can demonstrate the effects of tranexamic acid23 and FXIII supplementation.22
Correlation with clinical phenotype
A number of studies have demonstrated considerable heterogeneity in the baseline whole blood coagulation patterns among patients with verified FVIII activity levels <1%.19 Furthermore, data have illustrated that patients diagnosed with severe hemophilia A (FVIII:C <1%) associated with unusually good whole blood clotting profiles are associated with a less severe bleeding phenotype.24 The low tissue factor thrombelastography assay has also been used to illustrate different response patterns to various levels of FVIII concentrate replacement.
Prediction of response to bypassing agents
Both in vitro and in vivo studies have demonstrated the ability of thromboelastography to predict the clinical response to bypassing agents in patients with inhibitors.19,20,25 An early study that used TEG to individualize therapy in 3 inhibitor patients led to identification of the lack of effect of rFVIIa in 1 patient (which the patient’s mother had in fact suspected), while reducing the overall use of bypassing agents in 2 other patients led to more cost-effective care without compromising efficacy.20 This study also demonstrated the utility of TEG in assessing the effects of bypassing agents used in a combination for severe bleeds. In another study, thromboelastometry was used to improve the clinical performance of rFVIIa during surgery by demonstrating a need for fresh platelet concentrate to optimize the hemostatic effect of rFVIIa.26
Standardization of the Assays
A major challenge associated with the introduction of new laboratory tests is assay standardization. Assay standardization is needed to ensure adequate reproducibility both within the same laboratory (internal quality control) and with other laboratories performing the same assay (external quality assurance). For thrombin generation tests, the 2 major challenges are the numerous preanalytical variables involved in the procurement, processing, storage, and shipping of a plasma sample and the fact that there are several commercially available assays. With respect to the latter, it is important to understand that each device requires its own specific standardization. To date, much of the research with respect to hemophilia has used the calibrated automated thrombogram (CAT). Although the authors do not endorse any device, a standardized protocol has been developed for the CAT.27 Of note, these methods have been endorsed by the Scientific and Standardization Committee (SSC) of the International Society on Thrombosis and Haemostasis (ISTH), with a report expected in 2013. Unfortunately, this report will not address standardization issues with other thrombin generation devices.
For the viscoelastic devices, the main difficulty with standardization relates to the fact that the assay is best done on fresh whole blood, thus negating the ability to share the samples between multiple laboratories in an external quality assurance exercise. Having said this, the platelet function analyzer 100 (PFA-100) device, which also uses whole blood, has undergone external quality assurance standardization. This was done using locally collected blood with externally provided reagents and methods, suggesting that such standardization is feasible for thromboelastography as well.28 As for the thrombin generation tests, standardized methods for performing viscoelastic assays have been endorsed by the SSC, with a report expected in 2013. Once this report is published, it will be incumbent upon the researchers in this field to conduct an external quality assurance program, as was done for the PFA-100.
Controversies
Despite the accumulated research, several areas of controversy exist with respect to thrombin generation tests and viscoelastic tests that need to be reconciled prior to widespread clinical application. Although these assays are the closest to being implemented in the clinical setting, issues remain regarding the tissue source (whole blood vs PPP or PRP), the viscoelastic activators (extrinsic vs intrinsic activation), and the tissue factor source/provider and concentration if extrinsic activation is used. Because the preferred source of test sample for the thrombin generation test is PPP and the preferred source for the viscoelastic method is whole blood, this controversy becomes a de facto controversy of the assays themselves. There are certain advantages with each blood fraction. PPP can be frozen, stored, and shipped, which facilitates the ability to perform the test remotely both in terms of time (the sample can be stored and run when convenient) and place (the assay can be run on patients whose treatment center does not have access to the device). Last, thanks to the ability to store frozen plasma indefinitely, studies using stored plasma from biorepositories can be performed long after the samples have been collected. The main limitation of plasma-based assays is that they do not assess the contribution of cellular elements involved in coagulation, particularly platelets. In addition, the use of whole blood incorporates other cellular elements that may contribute to clot formation and requires less manipulation of the blood sample, making the assay substantially simpler and reducing the potential effect of preanalytic variables. Although one could construe such a discussion of the assay (and source tissue) comparison in a competitive fashion, the authors believe these assays should be viewed as complementary, with each providing different information. It is possible that, depending on the situation, one assay would be more advantageous than the other.
A second controversy involves the methods used for the TEG/ROTEM. First, it should be noted that although the devices have minor differences, the end result is essentially the same. The authors do not endorse any particular viscoelastic device. With respect to the methods, both a tissue factor (extrinsic activation) and kaolin (intrinsic activation) approach have been discussed. The basic argument in favor of the tissue factor method is that it is a relatively physiological approach, while kaolin is clearly not physiological. Favoring the kaolin approach is its simplicity and the fact that it does not rely on a biologic compound. Kaolin is provided in standard prefilled vials that require no manipulation, substantially simplifying performance of the assay. In contrast, tissue factor sources vary, are subject to lot-to-lot variation, and must be diluted several logs, all of which can lead to a loss of reliability in the results. Furthermore, there is currently no international tissue factor (TF) standard, and Parhami-Seren and colleagues have demonstrated how the specific activity of TF may vary according to its source.29 As an example of the potential difficulties that may be encountered, a multicenter study by Young et al that used the methods developed by Sørensen18 failed to demonstrate a dose–response effect of rFVIIa where previous studies had demonstrated such an effect.30 More recently, in vitro spiking studies comparing kaolin to 2 concentrations of recombinant human tissue factor (Innovin) demonstrated that the kaolin method is not only effective at discriminating the effects of rFVIIa and rFVIII but is perhaps even better than tissue factor at discriminating these effects.31,32 Yet another issue with the tissue factor method is the consideration of CTI to inhibit the intrinsic system. Although CTI has been used in some studies that incorporated the tissue factor method, it remains uncertain whether addition of CTI improves the reliability or validity of the assay. In addition, employing CTI adds yet another variable to the extrinsic factor method and also adds significantly to the cost. Moving forward, it is suggested that studies use both approaches given the perceived advantages of each and the potential that one may be better at discriminating between certain phenotypes of patients or at discriminating the effects of a potential therapy. From a practical standpoint, unless the TF method demonstrates superiority in discriminating phenotypes or responses to treatment, it is more likely that clinical laboratories will adopt the kaolin method because it is much simpler, is less expensive, and is the licensed method (in the United States).
A third controversy involves the tissue factor concentration that should be used. As discussed here, there are multiple potential sources of tissue factor. However, given a specific source, the concentration that should be used remains unclear. Although the majority of studies have used a 1:17 000 dilution of Innovin, which corresponds to approximately 0.35pM, other studies have used dilutions of 1:42 000 (∼0.1pM) and even 1:200 000 (∼0.035pM). Currently it is not clear which source of tissue factor should be used (although most studies have used Innovin) or which concentration should be used. As stated, in 2013, the SSC will publish specific recommendations to address this issue.
Future Directions
During the past 10 years, numerous studies using thrombin generation tests and viscoelastic tests in hemophilia have led to a detailed understanding of the preanalytical methods and performance of these assays. In addition, several pilot clinical studies have reported promising results, opening new perspectives on managing patients with hemophilia. Currently, one can envision 4 distinct areas where global assays could provide important data that could impact clinical decision making in hemophilia. These include defining an individual patient’s phenotype, individualizing therapy for patients without inhibitors on prophylaxis, individualizing bypassing agent therapy for management of bleeding and prevention of bleeding during surgery, and assessing the “hemostatic level” of novel FVIII and FIX molecules currently in clinical development.
With respect to defining the bleeding phenotype, some groups have reported a correlation between the thrombin-generating capacity of patients with severe hemophilia and their clinical bleeding phenotype.7-9 As mentioned previously, 10% to 15% of severe hemophiliacs exhibit a mild bleeding phenotype, and neither aPTT-based assays nor genotyping of patients can distinguish the mild bleeders from those with a more severe bleeding phenotype. A laboratory assay that can accurately predict the bleeding risk of each severe hemophilia patient may be of high clinical and economic value when deciding which patients should receive prophylaxis early in life.
Second, because global assays are sensitive to small amounts of thrombin (and thus FVIII or FIX in hemophilia patients), such laboratory tests may also lead to new possibilities for individually tailored prophylaxis regimens that are adapted to each patient’s personal needs.33 Several groups have reported preliminary data with thrombin generation assays that support this hypothesis.7,10,11 Ex vivo spiking of plasma samples with FVIII concentrates showed a clear dose-dependent effect that can be quite variable from one patient to another.11 In vivo pharmacokinetic studies of FVIII obtained after the infusion of a therapeutic dose of FVIII concentrate confirmed the previous ex vivo findings and reported different trough levels of thrombin generation in patients having similar plasma FVIII levels 24 hours after the FVIII infusion.7,10 The major practical application of such an approach would be the individualization of prophylaxis regimens that would potentially result in less frequent dosing, which would be more convenient and less expensive. It is possible (if not likely) that the newer, long-acting FVIII and FIX molecules in clinical trials will have even more interindividual differences in response and half-life given their different mechanisms of action (see discussion below) that would only enhance the need for a more tailored laboratory-based approach.
Third, the use of global assays to determine the efficacy of bypassing therapy is another important and active area of research. The optimal use of bypassing agents is hampered by a lack of laboratory assays to determine adequate dosing and monitor therapeutic efficacy. The ability to determine the most effective therapeutic option and the optimal dose and frequency of administration of a bypassing agent for the management of bleeding or surgery would represent a major advance. Both thrombin generation assays and thromboelastography have been evaluated for this purpose, and promising preliminary data have been published.10-13,20 The first prospective clinical assessment of the thrombin generation assay for dose tailoring and monitoring coagulation induced by rFVIIa and APCC in inhibitor patients undergoing surgical procedures has been published.13 This thrombin generation test-based strategy for the management of bypassing therapy in surgical settings showed that the assay represents a promising laboratory tool that can help physicians individually tailor and monitor hemostatic treatment in inhibitor patients. Considering that assays to specifically assess the activity of bypassing agents are neither available nor in development, this represents the area where the practical application of these assays is most pressing. For such patients, individually tailored regimens for surgery and bleeding should be urgently studied in laboratories that have the expertise to interpret the results.
Last, the field of hemophilia is about to enter a new era in which numerous new pharmacologic agents will be introduced. These include FVIII and FIX molecules that have been modified to improve their duration of action, as well as agents with novel mechanisms of action such as inhibition of endogenous tissue factor pathway inhibitor in order to exert a prohemostatic response. A major potential limitation to such new agents is the ability to accurately and simply measure their hemostatic effect by laboratory assays. Moreover, even if 1-stage clotting assays could measure the level of modified factors VIII and IX, it is expected that a specific standard will be required for each product. For the coagulation laboratory and the clinician this will add a degree of complexity that inherently will be, at best, time-consuming and costly and, at worst, could result in errors affecting patient outcomes. Because global assays simply measure either thrombin generation or clot formation, they should not be subject to the various modifications of the novel agents and as such have the potential to become an assay that can predict clinical efficacy regardless of which product is being administered to the patient. This will become an area of active research and could lead to a paradigm shift in the way factor replacement therapy is monitored. Finally, although novel replacement therapies will be available in the near future, other novel therapeutics may have new mechanisms of action (ie, not simply replacing what is missing) and such agents will obviously not be measurable by clotting factor assays. For such agents, global hemostatic assays have been demonstrated to be potentially effective methods for determining their effect in vitro and in animal models and will likely be the best way to measure their effect once these agents are available.
At this time, there is sufficient understanding of these assays to advance them from the setting of experimental research to the setting of clinical trials, with the ultimate goal of applying these assays in the clinic. In fact, with the explosion of novel clotting factors that have enhanced properties, some with a longer duration of action and others with a more potent hemostatic effect, now is the ideal time to begin incorporating global assays into clinical trials. Importantly, global assays may be the ideal method for monitoring the effect of these new therapeutics. Incorporation of these assays into such studies will allow for a correlation of the clinical response (still to be measured by validated, presumably subjective, measures) with an objective laboratory endpoint. These clinical studies should ultimately determine the potential clinical utility of these assays.
The future of hemophilia management must involve more objective measures of the outcomes of clinical interventions. Applying simple, inexpensive, reliable, and informative laboratory assays that can be correlated to the patient’s clinical outcome of should improve our understanding of the results of clinical trials and improve our ability to select the best and most cost-effective dose of factor replacement. This in turn should reduce the cost of care for patients with hemophilia while simultaneously improving their quality of life.
Acknowledgments
There was no specific funding supporting the efforts of the writing of this manuscript and medical writers were not used. K.B.-Z. acknowledges that she receives grant support from the National Institutes of Health (grant HL46703, project 5).
Authorship
Contribution: G.Y. organized and led the writing of the manuscript and wrote the first draft of the background, the section on thromboelastography, controversies, and future directions. B.S. wrote the first draft of the section on thrombin generation tests. K.B.-Z. wrote the first draft of the section on the physiology of hemostasis. Y.D. and C.N. edited the first draft. All authors contributed to the final edits of the manuscript. N.S.K. edited the manuscript and helped prepare it for submission. Y.D. provided Figures 1 and 2. G.Y. provided Figures 3 and 4.
Conflict-of-interest disclosure: B.S. is currently an employee of Baxter Biosciences; however, his contributions to the manuscript predate his acceptance of a position with that company. The remaining authors declare no competing financial interests.
Correspondence: Guy Young, MD, Children’s Hospital Los Angeles, 4650 Sunset Blvd, Los Angeles, CA 90027; e-mail:gyoung@chla.usc.edu.