Key Points
First report describing in vivo function of siglec-E as a negative regulator of neutrophil recruitment in acute lung inflammation.
Implications for the human functional ortholog, siglec-9, and its potential role in regulating inflammatory lung disease.
Abstract
Neutrophil entry into the lung tissues is a key step in host defense to bacterial and yeast infections, but if uncontrolled can lead to severe tissue damage. Here, we demonstrate for the first time that sialic acid binding Ig-like lectin E (siglec-E) functions to selectively regulate early neutrophil recruitment into the lung. In a model of acute lung inflammation induced by aerosolized lipopolysaccharide, siglec-E–deficient mice exhibited exaggerated neutrophil recruitment that was reversed by blockade of the β2 integrin, CD11b. Siglec-E suppressed CD11b “outside-in” signaling, because siglec-E–deficient neutrophils plated on the CD11b ligand fibrinogen showed exaggerated phosphorylation of Syk and p38 mitogen-activated protein kinase. Sialidase treatment of fibrinogen reversed the suppressive effect of siglec-E on CD11b signaling, suggesting that sialic acid recognition by siglec-E is required for its inhibitory function. Siglec-E in neutrophils was constitutively associated with the tyrosine phosphatase SHP-1 and may therefore function to constitutively dampen inflammatory responses of neutrophils. These data reveal that siglec-E is an important negative regulator of neutrophil recruitment to the lung and β2 integrin–dependent signaling. Our findings have implications for the human functional ortholog, siglec-9, and its potential role in regulating inflammatory lung disease.
Introduction
Neutrophil recruitment to the lung represents an important first line of defense against bacterial infections or inhaled environmental toxins. In contrast to many other tissues, neutrophil recruitment in the inflamed lung occurs mostly in the capillary beds rather than in the larger vessels such as postcapillary venules.1 Most lung capillaries are narrower than the diameter of neutrophils, resulting in neutrophil distortion and slow transit times.2 Following exposure to proinflammatory stimuli such as lipopolysaccharide (LPS), circulating neutrophils increase their cytoskeletal rigidity, and this can lead to trapping and arrest within the lung capillaries.3 In the presence of an appropriate chemokine gradient, neutrophils diapedese across the endothelial and epithelial barriers and enter the alveolar spaces,4 a process that in the case of LPS stimulation depends on the β2-integrin CD11b.5
Because the lung is under constant exposure to environmental challenge, setting appropriate thresholds for leukocyte adhesion and recruitment are crucial, both for maintaining normal lung homeostasis and permitting a rapid response to pathogenic threats or lung injury. Activated neutrophils release oxygen intermediates and proteolytic enzymes that efficiently kill pathogens and hasten the resolution of inflammation. However, bystander injury to normal tissues induced by neutrophil infiltration is an unavoidable feature of the local inflammatory response.
To date, there have been few reports describing negative regulators in neutrophil biology. Many inhibitory receptors contain immunoreceptor tyrosine-based inhibitory motifs (ITIMs) and play fundamental roles in the negative regulation of a wide range of immune and inflammatory responses via the recruitment of tyrosine phosphatases SHP-1 and SHP-2.6 A key role for SHP-1 in negative regulation of neutrophil function has been shown by the overexpansion and inappropriate activation of granulocyte populations in SHP-1–deficient motheaten mice.7,8 Moreover, via association with SHP-1, Ly49Q has been reported to regulate inappropriate activation and adhesion of neutrophils by preventing focal complex formation.9 Other recent reports describe GDF-15 as a novel inhibitor of neutrophil recruitment by preventing chemokine triggered β2-integrin activation and clustering10 and Btk as a “gatekeeper,” important for preventing the priming of neutrophils and modulation of their production of reactive oxygen species to prevent death by apoptosis.11
Siglecs are type 1 membrane proteins of the Ig superfamily that mediate sialic acid–dependent interactions with ligands via their N-terminal Ig domain.12 Siglec-E is a murine inhibitory receptor expressed constitutively on neutrophils and is induced on macrophages by LPS.13,14 Following phosphorylation by Src-family kinases, the ITIMs of siglec-E are able to recruit Src homology domain-containing protein tyrosine phosphatases, SHP-1 and SHP-2, in both transfected cell lines15 and in LPS-activated macrophages.13 However, the functional role of siglec-E in vivo and its signaling pathways in neutrophils remain unknown. Our data reveal siglec-E as an important negative regulator of acute lung inflammatory responses and β2 integrin–dependent signaling in neutrophils.
Materials and methods
For further details regarding materials, see supplemental Materials and Methods on the Blood website.
Generation of siglec-E–deficient mice
Siglec-E−/− mice were generated on a 129/Sv background by gene targeting as shown in Figure 1A. Details of library screening and clone manipulation are provided in supplemental Materials and Methods. Siglec-E−/− mice were backcrossed to the C57BL/6 background for >15 generations. Generation of the siglec-E R126D mutant mouse was performed by Taconic Artemis using C57Bl/6 ES cells, and the targeting strategy is illustrated in Figure 1B. All mice were bred and maintained under specific pathogen-free conditions at the University of Dundee. All procedures were carried out with institutional ethics approval and were performed in accordance with the UK 1986 Animals (Scientific Procedures) Act.
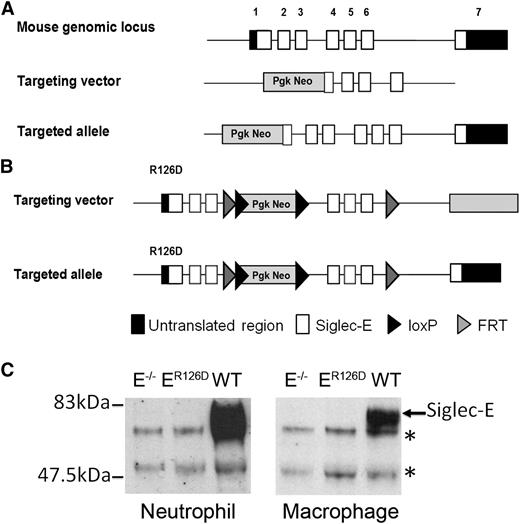
Generation of siglec-E–deficient mice. (A) Schematic representation of Siglec-E locus, the gene targeting vector, and the predicted mutated Siglec-E gene. (B) Targeting vector to introduce R126D mutation in exon 1 (that contains the sialic acid binding site), with the aim of generating a full-length protein that lacks the ability to bind sialic acid. (C) Lysates from bone marrow neutrophils or bone marrow–derived macrophages cultured for 3 days in the presence of 100 ng/mL LPS to induce siglec-E were immunoprecipitated with sheep anti-mouse siglec-E IgG and subsequently immunoblotted with sheep anti-mouse siglec-E antiserum followed by rabbit anti-sheep IgG-HRP conjugate. *An upper nonspecific band and a lower heavy chain band were common to all samples.
Generation of siglec-E–deficient mice. (A) Schematic representation of Siglec-E locus, the gene targeting vector, and the predicted mutated Siglec-E gene. (B) Targeting vector to introduce R126D mutation in exon 1 (that contains the sialic acid binding site), with the aim of generating a full-length protein that lacks the ability to bind sialic acid. (C) Lysates from bone marrow neutrophils or bone marrow–derived macrophages cultured for 3 days in the presence of 100 ng/mL LPS to induce siglec-E were immunoprecipitated with sheep anti-mouse siglec-E IgG and subsequently immunoblotted with sheep anti-mouse siglec-E antiserum followed by rabbit anti-sheep IgG-HRP conjugate. *An upper nonspecific band and a lower heavy chain band were common to all samples.
Airway inflammation model
Groups of gender- and age-matched mice were exposed to an aerosol of LPS at a concentration of 0.1 mg/mL for 30 minutes.16 Bronchoalveolar lavage (BAL) and lung digestion were performed as described,17 and cell populations were identified either by morphology on stained cytocentrifuge slides or by flow cytometry as described below. To investigate neutrophil margination in the lung, mice were administered with 50 µg LPS intraperitoneally. To investigate the functional role for CD11b, immediately before LPS aerosol, mice were injected intravenously with 100 μg purified 5C6 anti-CD11b antibody18 or an equivalent amount of rat IgG.
Generation of radiation bone marrow chimeras
Bone marrow cells from CD45.1/CD45.2 wild-type (WT) mice or siglec-E−/− mice were injected intravenously into irradiated (11 Gy) WT CD45.1 or siglec-E−/− (CD45.2) recipient mice. Eight weeks after reconstitution, mice were exposed to aerosolized LPS and lung tissue digest cells were analyzed by flow cytometry. Neutrophils were labeled with antibodies to Gr-1, CD11b, and siglec-E (generated in house). Fc receptor binding was blocked with 2.4G2 before surface staining. After staining, cells were fixed in 1% formaldehyde. Data were collected using a LSR Fortessa (BD Biosciences, Oxford, UK) and analyzed using Flowjo 7.5 software (Treestar, Ashland, OR).
Neutrophil activation assays
In vitro stimulation.
Bone marrow cells (1 × 106) or 200 μL whole blood were incubated for 30 minutes at 37°C with stimuli. Neutrophils were delineated with antibodies to Gr-1 and siglec-E, and activation was assessed by levels of CD11b and CD62L by flow cytometry as described earlier.
Chemotaxis assays.
Bone marrow cells were seeded as a 1:1 mix (3 × 106 total cells in Hanks balanced salt solution containing Ca2+, Mg2+, 30 mM N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid, and 0.1% bovine serum albumin) into the upper well of transwell chemotaxis chambers. The number of neutrophils migrating to the lower well in response to CXCL1 or CXCL2 over 30 minutes at 37°C was assessed by flow cytometry.
Cell adhesion assays.
Assays were performed as described19 using fibrinogen (100 μg/mL) as CD11b ligand.
Biochemical analysis
Detection of siglec-E in neutrophils and macrophages.
Lysates were prepared from bone marrow neutrophils or LPS-stimulated bone marrow–derived macrophages.13 Following immunoprecipitations using sheep polyclonal anti–siglec-E IgG, western blots were probed with sheep anti–siglec-E antiserum followed by rabbit anti-sheep IgG conjugated to horseradish peroxidase (HRP). Proteins were detected by autoradiography using enhanced chemiluminescence (ECL) substrate.
Analysis of Syk and p38 MAP kinase phosphorylation.
Bone marrow cells (3 × 106) were centrifuged onto 6-well tissue culture plates either uncoated or coated with ligands.20 Cells were lysed in 50 mM Tris-HCl, 150 mM NaCl, and 1% NP-40 with protease inhibitors. Immunoblots were probed with antibodies against phospho- and total Syk or phospho- and total p38 mitogen-activated protein (MAP) kinase followed by HRP-conjugated goat anti-rabbit IgG. Proteins were detected by autoradiography, and Image J (http://rsbweb.nih.gov/ij) was used for densitometric analysis of autoradiographs. In some experiments, coated wells were treated with 50 mM sodium acetate buffer, pH 5.5, containing 1 mM CaCl2, with or without 0.17 U/mL Vibrio cholerae sialidase for 1 hour at 37°C before neutrophil plating.
SHP-1 and SHP-2 coimmunoprecipitation with siglec-E.
Bone marrow cells were plated as described above or stimulated in suspension with LPS (10 µg/mL). Lysates were prepared as above and subjected to immunoprecipitation with anti–siglec-E monoclonal antibody.13 Immunoblots were probed with antibodies to SHP-1, SHP-2, siglec-E (sheep polyclonal), and phospho-tyrosine followed by HRP-conjugated anti-rabbit or anti-sheep secondary antibodies before ECL autoradiography.
Fibrinogen dot blot assay.
Fibrinogen was spotted onto a nitrocellulose membrane and blocked with 5% milk in Tris-buffered saline containing 0.05% Tween 20. Membranes were treated with or without sialidase as above and incubated with siglec-E-Fc/anti-Fc peroxidase precomplexes or anti-human fibrinogen antibody for 1 hour at room temperature. Binding was detected by ECL chemiluminescence, and image analysis was performed using AIDA Image Analyzer software (Raytest GmbH, Straubenhardt, Germany).
Fluorescence microscopy
Bone marrow cells were incubated on fibrinogen-coated coverslips for 20 minutes at 37°C, fixed with 3.7% formaldehyde, and stained with sheep polyclonal anti–siglec-E IgG conjugated to FluoProbe 547H and/or anti–CD11b-Alexa488 antibody. Cells were mounted in 4,6 diamidino-2-phenylindole and analyzed by confocal microscopy. Excitation wavelengths were 405, 488, and 555 nm, and emission wavelengths maxima were 493/519 nm and 557/574 nm. Image analysis was performed using Volocity software (PerkinElmer, Waltham, MA).
Data analysis
Data are expressed as mean ± standard error of the mean (SEM). Statistical significance between groups was tested using a Mann-Whitney U test or a paired t test for biochemical analysis. A P value of <.05 was considered significant.
Results
Generation and characterization of siglec-E–deficient mice
To study the role of siglec-E in neutrophil recruitment to the lung, we generated siglec-E–deficient mice by gene targeting using two different approaches (Figure 1). First, exons 1 and 2 were replaced with a neomycin cassette in 129 embryonic stem cells (Figure 1A). The resulting siglec-E–deficient mice were backcrossed onto the C57Bl/6 background and are referred to as siglec-E−/− mice. Second, a targeted mutation was introduced into the Siglec-E gene in C57Bl/6 embryonic stem cells to destroy the sialic acid binding site by changing arginine 126 to aspartic acid (Figure 1B). The ensuing mice are referred to as siglec-ER126D. Both siglec-E−/− and siglec-ER126D mouse lines were generated at normal Mendelian frequencies, were viable and displayed no gross differences compared with their respective WT controls. Analysis of leukocyte subpopulations in WT, siglec-E−/−, and siglec-ER126D mice showed no alterations in either the percentage or number of each cell type analyzed (supplemental Table 1). This analysis also confirmed the previously reported14 expression of siglec-E on neutrophils and CD11c+ splenic dendritic cells. Siglec-E was not detected on mature T and B cells, NKp46+ natural killer cells (supplemental Figure 1), and platelets (data not shown).
As expected, siglec-E protein was undetectable in immunoprecipitates from siglec-E−/− bone marrow neutrophils and LPS-induced bone marrow–derived macrophages (Figure 1C). Lack of siglec-E expression was further confirmed by flow cytometry of neutrophils, dendritic cells, and macrophages from siglec-E−/− mice using specific monoclonal and polyclonal Abs (supplemental Figure 1 and data not shown). Surprisingly, siglec-E protein was also undetectable in siglec-ER126D mice (Figure 1C). This was associated with low or undetectable levels of siglec-E mRNA in bone marrow neutrophils and LPS-induced macrophages (data not shown), suggesting that gene transcription in siglec-ER126D mice was severely affected by the targeting strategy used. Because both siglec-E−/− and siglec-ER126D strains of mice were deficient in siglec-E they were used interchangeably throughout the study, with similar results in all experiments.
Siglec-E is a negative regulator of acute pulmonary neutrophil recruitment
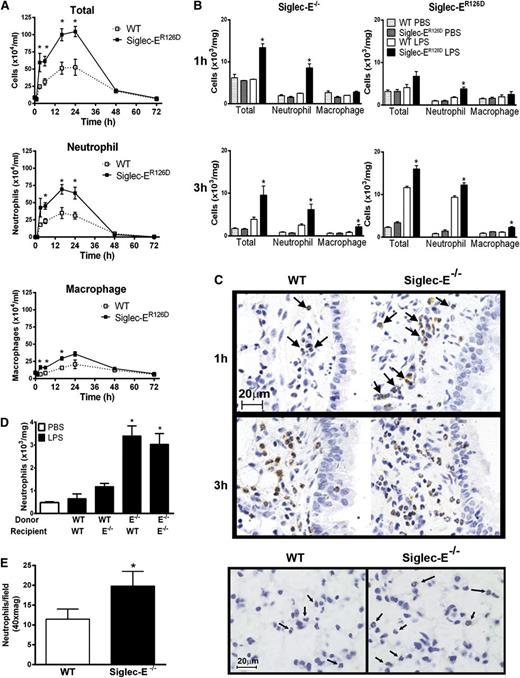
Using a well-characterized model of aerosolized LPS-induced acute lung airway inflammation,16 neutrophil recruitment to the airways was found to be more rapid and of greater magnitude in siglec-E–deficient mice in comparison with WT controls (Figure 2). This increased inflammatory response remained elevated over a time course of up to 24 hours, after which neutrophil numbers returned to levels comparable to WT mice (Figure 2A). Neutrophil recruitment was also analyzed at early time points in collagenase-digested lung tissue when cellular infiltrates were undetectable in BAL. We observed accelerated neutrophil recruitment in the lung tissue of siglec-E–deficient mice at 1 hour after LPS, whereas WT mice showed little early cellular changes (Figure 2B-C). Numbers of infiltrating macrophages were also elevated in the airways of siglec-E–deficient mice exposed to LPS (Figure 2A-B).
LPS-induced airway inflammation in siglec-E–deficient mice. Total and differential cell counts in (A) bronchoalveolar lavage and (B) lung tissue digests from WT mice and siglec-ER126D mice following aerosolized LPS. Values are expressed as means ± SEM; n = 4-6 per group; and *P < .05, Mann-Whitney U test compared with WT mice. (C) Paraffin-embedded lung tissue sections were stained with a specific antibody against myeloperoxidase to identify neutrophils (arrows). Scale bars represent 20 µm on images acquired with an Axioskop Zeiss microscope (63× PlanNeoFluor/1.25 objective) equipped with an Axiocam digital camera (Carl Zeiss Microscopy LLC, Thornwood, New York). Data are representative of n = 4-6 per group. (D) Eight weeks after reconstitution, radiation bone marrow chimeric mice were exposed to aerosolized LPS. One hour after LPS, cells were isolated from lung tissue digests, and numbers of neutrophils were determined by flow cytometry. Data are expressed as means ± SEM; n = 4-5 per group; and *P < .05, Mann-Whitney U test compared with mice reconstituted with WT cells. (E) Increased margination of neutrophils in the lung tissue of siglec-E–deficient mice 4 hours after 50 μg intraperitoneal LPS. Paraffin-embedded lung tissue sections were stained with a specific antibody against myeloperoxidase to identify neutrophils in the capillary bed (arrows; scale bars represent 20 µm). Neutrophils were enumerated in 10 random fields per section. Values are expressed as means ± SEM; n = 5 per group; and *P < .05, Mann-Whitney U test compared with WT mice.
LPS-induced airway inflammation in siglec-E–deficient mice. Total and differential cell counts in (A) bronchoalveolar lavage and (B) lung tissue digests from WT mice and siglec-ER126D mice following aerosolized LPS. Values are expressed as means ± SEM; n = 4-6 per group; and *P < .05, Mann-Whitney U test compared with WT mice. (C) Paraffin-embedded lung tissue sections were stained with a specific antibody against myeloperoxidase to identify neutrophils (arrows). Scale bars represent 20 µm on images acquired with an Axioskop Zeiss microscope (63× PlanNeoFluor/1.25 objective) equipped with an Axiocam digital camera (Carl Zeiss Microscopy LLC, Thornwood, New York). Data are representative of n = 4-6 per group. (D) Eight weeks after reconstitution, radiation bone marrow chimeric mice were exposed to aerosolized LPS. One hour after LPS, cells were isolated from lung tissue digests, and numbers of neutrophils were determined by flow cytometry. Data are expressed as means ± SEM; n = 4-5 per group; and *P < .05, Mann-Whitney U test compared with mice reconstituted with WT cells. (E) Increased margination of neutrophils in the lung tissue of siglec-E–deficient mice 4 hours after 50 μg intraperitoneal LPS. Paraffin-embedded lung tissue sections were stained with a specific antibody against myeloperoxidase to identify neutrophils in the capillary bed (arrows; scale bars represent 20 µm). Neutrophils were enumerated in 10 random fields per section. Values are expressed as means ± SEM; n = 5 per group; and *P < .05, Mann-Whitney U test compared with WT mice.
Increasing the concentration of aerosolized LPS from the standard dose of 0.1 to 1.0 mg/mL led to an even greater inhibitory effect of siglec-E on neutrophil recruitment (supplemental Figure 2A). This suggests that the dampening effect of siglec-E is dominant and not overridden at high concentrations of LPS. Reciprocal radiation bone marrow chimeras confirmed that the enhanced airway inflammation was caused by the absence of siglec-E expression on hematopoietic cells rather than environmental cells such as endothelial and epithelial cells that remain of host origin (Figure 2D). Following systemic injection of LPS, neutrophils transiently adhere to vascular endothelium of the lung but do not undergo transmigration.21 Following intraperitoneal LPS, we observed a clear increase in neutrophil numbers in the lung tissue capillary bed of siglec-E–deficient mice compared with WT controls (Figure 2E), demonstrating that siglec-E negatively regulates the early steps of neutrophil recruitment to the lung.
We also analyzed the response to ultrapure LPS-free zymosan that signals through toll-like receptor (TLR)-2 and dectin-122 as opposed to TLR-4 (and to a lesser degree TLR-2) in the case of LPS (supplemental Figure 2B). Following intranasal administration of zymosan, lung neutrophil recruitment was elevated in siglec-E–deficient mice, showing that the exaggerated inflammatory responses observed in the absence of siglec-E were not exclusive to LPS. We also analyzed thioglycollate-induced peritonitis (supplemental Figure 3A) and acute inflammation in an air pouch model using tumor necrosis factor-α23 or Staphylococcus aureus24 as a stimulant (supplemental Figure 3B). However, neutrophil recruitment was comparable between WT and siglec-E–deficient mice. These results indicate that the siglec-E–dependent suppression of neutrophil recruitment is selective for the lung.
Siglec-E negatively regulates CD11b-dependent adhesive function in vivo
In view of the previously suggested role of siglec-E as a negative regulator of LPS-induced cytokine production,13 we asked whether the exaggerated lung neutrophil recruitment in siglec-E–deficient mice was caused by increased chemokine production and/or chemotactic responses. As expected, we observed a significant LPS-dependent increase in levels of both CXCL1 and CXCL2 in serum, BAL, and lung tissue homogenates, but there were no differences in levels of chemokines between WT and siglec-E–deficient mice (Figure 3A). Similarly, there were no differences in chemotactic responses to CXCL1 and CXCL2 in transwell chemotaxis assays (Figure 3B). Together, these data argue against a defect in either chemokine production or neutrophil chemotactic responses in siglec-E–deficient mice.
Chemotactic responses and requirement for CD11b in exaggerated neutrophil recruitment of siglec-E–deficient mice exposed to LPS. (A) CXCL1 and CXCL2 levels were measured in serum, bronchoalveolar lavage (BAL) fluid, and lung tissue homogenate by enzyme-linked immunosorbent assay from WT mice and siglec-ER126D mice 30 minutes after LPS aerosol. Data are expressed as means ± SEM; n = 4-5 per group. (B) A 1:1 mix of WT and siglec-E−/− mice bone marrow cells were placed into transwell chemotaxis chambers, and the number of cells migrating was determined by flow cytometry. Data are expressed as means ± SEM of triplicate wells. (C) Mice were injected intravenously with 100 µg anti-CD11b antibody (5C6 clone 1) or rat IgG immediately preceding exposure to aerosolized LPS and cellular recruitment analyzed at 3 hours in BAL or in lung tissue digests (Lung). Data are expressed as means ± SEM; n = 4-9 per group from 2 independent experiments; and *P < .05, Mann-Whitney U test compared with WT mice.
Chemotactic responses and requirement for CD11b in exaggerated neutrophil recruitment of siglec-E–deficient mice exposed to LPS. (A) CXCL1 and CXCL2 levels were measured in serum, bronchoalveolar lavage (BAL) fluid, and lung tissue homogenate by enzyme-linked immunosorbent assay from WT mice and siglec-ER126D mice 30 minutes after LPS aerosol. Data are expressed as means ± SEM; n = 4-5 per group. (B) A 1:1 mix of WT and siglec-E−/− mice bone marrow cells were placed into transwell chemotaxis chambers, and the number of cells migrating was determined by flow cytometry. Data are expressed as means ± SEM of triplicate wells. (C) Mice were injected intravenously with 100 µg anti-CD11b antibody (5C6 clone 1) or rat IgG immediately preceding exposure to aerosolized LPS and cellular recruitment analyzed at 3 hours in BAL or in lung tissue digests (Lung). Data are expressed as means ± SEM; n = 4-9 per group from 2 independent experiments; and *P < .05, Mann-Whitney U test compared with WT mice.
Next, we considered the possibility that siglec-E was acting as a negative regulator of neutrophil adhesion to lung capillary endothelium after LPS exposure. The β2 integrin CD11b is required for neutrophil recruitment to the lungs of LPS-exposed mice.5 Injection of mice with a blocking anti-CD11b antibody significantly reduced neutrophil numbers in BAL and lung tissue digests in both WT and siglec-E–deficient mice and reversed the elevated neutrophil numbers in siglec-E–deficient mice back to levels seen in antibody-treated WT mice (Figure 3C). Taken together with the increased margination of neutrophils observed in siglec-E–deficient mice following intraperitonal LPS (Figure 2E), these results suggest that siglec-E negatively regulates CD11b-dependent adhesive function in vivo.
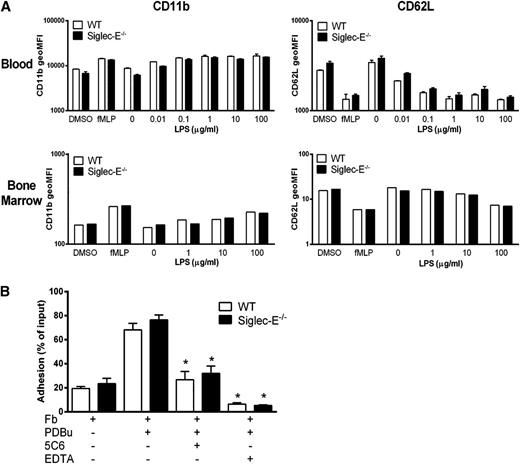
As activated neutrophils rapidly upregulate CD11b and shed CD62L after activation,25,26 we measured both markers on blood and bone marrow neutrophils before and after activation (Figure 4A). Freshly isolated neutrophils expressed similar levels of CD11b and CD62L (data not shown). After exposure to LPS or formyl-methionyl-leucyl-phenylalanine (fMLP), no differences were seen in either CD11b up-regulation or CD62L shedding compared with siglec-E–deficient and WT neutrophils in dose-response assays (Figure 4A). Although CD11b levels were unaltered in siglec-E–deficient neutrophils, it was important to see if CD11b was in a higher affinity state.27 As CD11b “affinity reporter” antibodies are not available in mice, the relative binding strength of this integrin was evaluated in short-term adhesion assays using fibrinogen as a CD11b ligand (Figure 4B). A low level of baseline adhesion was observed with unstimulated cells that was increased following integrin activation with phorbol esters and significantly reversed with anti-CD11b antibody or EDTA to block integrin function, but there were no differences between WT and siglec-E–deficient neutrophils (Figure 4B). In conclusion, these experiments indicate that siglec-E does not influence CD11b levels or binding strength either at baseline or after neutrophil activation.
Siglec-E does not regulate “inside-out” signaling of CD11b in neutrophils. (A) Whole blood or bone marrow cells from WT and siglec-E−/− mice, were incubated for 30 minutes at 37°C with the indicated stimuli. Surface expression of CD11b and CD62L on mature neutrophils (SSCintGr-1hiCD11b+) was assessed by flow cytometry. Data are expressed as mean ± standard deviation; n = 3 for blood and for bone marrow as the mean, representative of 2 independent experiments. (B) Adhesion of WT and siglec-E−/− bone marrow cells to fibrinogen (Fb) in the presence and absence of PDBu. To investigate integrin-dependent adhesion, cells were pretreated with 20 μg/mL anti-CD11b antibody (5C6 clone 1) or 10 mM EDTA 20 minutes before plating. Data are expressed as mean ± SEM with 5 replicates for each data point and are representative of 2 independent experiments.
Siglec-E does not regulate “inside-out” signaling of CD11b in neutrophils. (A) Whole blood or bone marrow cells from WT and siglec-E−/− mice, were incubated for 30 minutes at 37°C with the indicated stimuli. Surface expression of CD11b and CD62L on mature neutrophils (SSCintGr-1hiCD11b+) was assessed by flow cytometry. Data are expressed as mean ± standard deviation; n = 3 for blood and for bone marrow as the mean, representative of 2 independent experiments. (B) Adhesion of WT and siglec-E−/− bone marrow cells to fibrinogen (Fb) in the presence and absence of PDBu. To investigate integrin-dependent adhesion, cells were pretreated with 20 μg/mL anti-CD11b antibody (5C6 clone 1) or 10 mM EDTA 20 minutes before plating. Data are expressed as mean ± SEM with 5 replicates for each data point and are representative of 2 independent experiments.
Siglec-E suppresses CD11b-dependent Syk and p38 MAP kinase phosphorylation
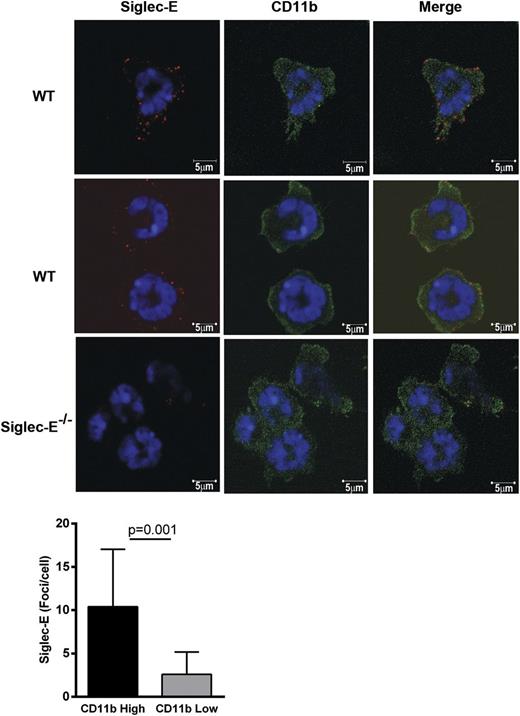
We next explored the possibility that siglec-E modulates CD11b “outside-in” signaling that can result in Src family kinase–dependent Syk activation and adhesion strengthening.28 Confocal microscopy of neutrophils adherent to fibrinogen showed that siglec-E was present on the cell surface in a punctate manner. Interestingly, siglec-E foci were more frequently seen in areas of intense CD11b staining than in areas of lower CD11b intensity (Figure 5). In western blotting analysis of whole cell lysates, the absence of siglec-E led to a twofold increase in Y317Syk phosphorylation at 20 minutes after plating of neutrophils on fibrinogen (Figure 6A), but did not affect phosphorylation of pY346Syk and pY519/520Syk (supplemental Figure 4). We also observed a parallel increase in phospho-p38 MAP kinase in siglec-E–deficient neutrophils (Figure 6A), but there was no effect of siglec-E deficiency on extracellular signal-regulated kinase or c-Jun N-terminal kinase MAP kinase phosphorylation (data not shown). The suppressive effect of siglec-E on Syk and p38 MAP kinase phosphorylation was selective because no differences were observed between WT and siglec-E–deficient neutrophils plated on IgG/anti-IgG complexes to trigger Fc receptors (Figure 6A) or after exposure to LPS (data not shown).
Siglec-E is preferentially localized in areas of high CD11b staining on neutrophils spreading over fibrinogen. WT and siglec-E−/− bone marrow cells were plated onto fibrinogen-coated coverslips for 20 minutes at 37°C. Neutrophils were fixed with 3.7% formaldehyde in phosphate-buffered saline and stained with polyclonal anti–siglec-E antibody conjugated to FluoProbe 547H and/or anti–CD11b-Alexa488 antibody. The cells were mounted in the presence of 4,6 diamidino-2-phenylindole to distinguish neutrophil nuclear morphology and were analyzed by confocal microscopy (LSM 700 microscope and Zen 2009 software; Carl Zeiss). Images were collected with an α-Plan-Apochromat × 100 NA 1.46 objective. Scale bars represent 5 µm. Image analysis was performed using Volocity software. The number of siglec-E foci per cell was quantified in areas of high CD11b staining compared with areas of low CD11b staining. High CD11b intensity was set to 1 to 3.3 standard deviations from the mean intensity, and low CD11b intensity was set to 0 to 1 standard deviations. The number of siglec-E foci was measured automatically in each region. Data are expressed as mean ± SEM from 10 images from 2 independent experiments containing ≥4 cells per image; *P < .05, paired t test.
Siglec-E is preferentially localized in areas of high CD11b staining on neutrophils spreading over fibrinogen. WT and siglec-E−/− bone marrow cells were plated onto fibrinogen-coated coverslips for 20 minutes at 37°C. Neutrophils were fixed with 3.7% formaldehyde in phosphate-buffered saline and stained with polyclonal anti–siglec-E antibody conjugated to FluoProbe 547H and/or anti–CD11b-Alexa488 antibody. The cells were mounted in the presence of 4,6 diamidino-2-phenylindole to distinguish neutrophil nuclear morphology and were analyzed by confocal microscopy (LSM 700 microscope and Zen 2009 software; Carl Zeiss). Images were collected with an α-Plan-Apochromat × 100 NA 1.46 objective. Scale bars represent 5 µm. Image analysis was performed using Volocity software. The number of siglec-E foci per cell was quantified in areas of high CD11b staining compared with areas of low CD11b staining. High CD11b intensity was set to 1 to 3.3 standard deviations from the mean intensity, and low CD11b intensity was set to 0 to 1 standard deviations. The number of siglec-E foci was measured automatically in each region. Data are expressed as mean ± SEM from 10 images from 2 independent experiments containing ≥4 cells per image; *P < .05, paired t test.
Siglec-E is a negative regulator of CD11b-dependent phosphorylation of Syk and p38 MAP kinase. (A) Increased phosphorylation of Syk and p38 MAP kinase in siglec-E−/− cells following adhesion to fibrinogen. WT and siglec-E−/− bone marrow cells were plated at 37°C under the different conditions and times indicated, and whole cell lysates were immunoblotted with antibodies against phopho-Y317Syk, total Syk, phospho-p38, and total p38. As a negative control, cells were treated with the Src family kinase inhibitor PP2 (20 µM). As a positive control, cells were treated with freshly prepared pervanadate (PV) to inhibit cellular tyrosine phosphatases. Phospho-Syk and phospho-p38 signals for each sample were normalized to their respective total Syk or total p38 signals. Data are representative of 3 independent experiments. Bar charts show densitometry expressed as means ± SEM; n = 3 per group; and *P < .05, paired t test compared with WT control. (B) Siglec-E binds fibrinogen in a sialic acid–dependent manner. Siglec-E-Fc precomplexes were incubated on fibrinogen pretreated with or without sialidase. Bar charts show densitometry expressed as means ± SEM; n = 3 per group; and *P < .05, paired t test compared with untreated membranes. (C) Sialidase treatment of fibrinogen reverses siglec-E–dependent inhibition of phopho-Y317Syk. Wells were coated with fibrinogen or IgG/anti-IgG complexes and either left untreated (No buffer) or were treated with V. cholerae sialidase (Sialidase) or sialidase buffer alone (Buffer) for 60 minutes. After blocking with 10% fetal calf serum, bone marrow cells were allowed to adhere for 20 minutes. Lysates were immunoblotted with antibodies to phospho-Y317Syk antibody and total Syk. Bar charts show densitometry expressed as means ± SEM; n = 4 per group; and *P < .05, paired t test compared with WT control.
Siglec-E is a negative regulator of CD11b-dependent phosphorylation of Syk and p38 MAP kinase. (A) Increased phosphorylation of Syk and p38 MAP kinase in siglec-E−/− cells following adhesion to fibrinogen. WT and siglec-E−/− bone marrow cells were plated at 37°C under the different conditions and times indicated, and whole cell lysates were immunoblotted with antibodies against phopho-Y317Syk, total Syk, phospho-p38, and total p38. As a negative control, cells were treated with the Src family kinase inhibitor PP2 (20 µM). As a positive control, cells were treated with freshly prepared pervanadate (PV) to inhibit cellular tyrosine phosphatases. Phospho-Syk and phospho-p38 signals for each sample were normalized to their respective total Syk or total p38 signals. Data are representative of 3 independent experiments. Bar charts show densitometry expressed as means ± SEM; n = 3 per group; and *P < .05, paired t test compared with WT control. (B) Siglec-E binds fibrinogen in a sialic acid–dependent manner. Siglec-E-Fc precomplexes were incubated on fibrinogen pretreated with or without sialidase. Bar charts show densitometry expressed as means ± SEM; n = 3 per group; and *P < .05, paired t test compared with untreated membranes. (C) Sialidase treatment of fibrinogen reverses siglec-E–dependent inhibition of phopho-Y317Syk. Wells were coated with fibrinogen or IgG/anti-IgG complexes and either left untreated (No buffer) or were treated with V. cholerae sialidase (Sialidase) or sialidase buffer alone (Buffer) for 60 minutes. After blocking with 10% fetal calf serum, bone marrow cells were allowed to adhere for 20 minutes. Lysates were immunoblotted with antibodies to phospho-Y317Syk antibody and total Syk. Bar charts show densitometry expressed as means ± SEM; n = 4 per group; and *P < .05, paired t test compared with WT control.
As fibrinogen is a sialylated glycoprotein,29 we investigated whether siglec-E–sialic acid interactions were required for the suppression of CD11b-dependent Y317Syk phosphorylation. Using dot blot analysis, we first demonstrated that siglec-E can bind to fibrinogen in a sialic acid–dependent manner (Figure 6B). We then showed that pretreatment of fibrinogen with sialidase reversed the inhibitory effect of siglec-E on Y317Syk phosphorylation (Figure 6C). In conclusion, these results demonstrate that sialic acid–dependent interactions of siglec-E are required for inhibitory signaling.
Siglec-E associates with SHP-1 in the absence of tyrosine phosphorylation
Because recruitment of SHP-1 and SHP-2 could be important in the siglec-E–dependent suppression of Syk phosphorylation in neutrophils, we investigated their association with siglec-E (Figure 7). Interestingly, siglec-E was seen to be constitutively associated with SHP-1 in neutrophils, even in the absence of detectable siglec-E tyrosine phosphorylation, but SHP-2 association was not observed (Figure 7). Stimulation of neutrophils with pervanadate or LPS induced robust tyrosine phosphorylation of siglec-E, but this only modestly increased SHP-1 association and did not lead to detectable SHP-2 association (Figure 7). In conclusion, these results demonstrate that siglec-E in neutrophils is constitutively associated with SHP-1 and provide a potential explanation for siglec-E–dependent suppression of Syk phosphorylation.
Siglec-E associates with SHP-1 in the absence of tyrosine phosphorylation. WT and siglec-E−/− bone marrow cells were plated on fibrinogen or stimulated in suspension with either pervanadate (PV) or with LPS (10 µg/mL) for the indicated time periods at 37°C. Lysates were immunoprecipitated with anti–siglec-E antibody followed by immunoblotting with anti–siglec-E, anti–SHP-1, anti–SHP-2, and anti-pY antibodies. As a positive control for siglec-E and SHP-2, lysates from WT and siglec-E–transfected CHO cells were analyzed in parallel. Data are representative of 3 independent experiments.
Siglec-E associates with SHP-1 in the absence of tyrosine phosphorylation. WT and siglec-E−/− bone marrow cells were plated on fibrinogen or stimulated in suspension with either pervanadate (PV) or with LPS (10 µg/mL) for the indicated time periods at 37°C. Lysates were immunoprecipitated with anti–siglec-E antibody followed by immunoblotting with anti–siglec-E, anti–SHP-1, anti–SHP-2, and anti-pY antibodies. As a positive control for siglec-E and SHP-2, lysates from WT and siglec-E–transfected CHO cells were analyzed in parallel. Data are representative of 3 independent experiments.
Discussion
Using a model of LPS-induced acute airway inflammation, we demonstrate that siglec-E functions as a nonredundant negative regulator of CD11b-dependent neutrophil recruitment into the lung. We ruled out the possibility that siglec-E expression on nonhematopoietic cells was important and found no evidence for siglec-E–dependent modulation of chemokine production or chemotaxis. Rather, our results indicate that siglec-E plays a key role in regulating CD11b-dependent adhesive interactions of neutrophils with the lung capillary endothelium. First, we showed that the exaggerated neutrophil recruitment was reversed with a blocking CD11b antibody. Second, after systemic administration of LPS, the number of marginating neutrophils in the lung was clearly increased in siglec-E–deficient mice. The lung uses an atypical pathway for neutrophil recruitment involving the capillary beds rather than postcapillary venules and appears to be less dependent on vascular E- and P-selectins than other tissues.30,31 The lack of siglec-E influence on neutrophil recruitment to the peritoneal cavity and air pouch may therefore be caused by the stronger dependence on selectin-mediated pathways in these tissues that could mask the influence of siglec-E. Further studies are required to determine whether siglec-E–dependent inhibition of neutrophil recruitment is selective for the lung.
Similar to many other integrins, CD11b can be regulated by “inside-out” signaling in response to diverse stimuli, leading to both increased surface expression from intracellular pools and increased binding affinity of integrin for ligands.25,28 However, we found no evidence that siglec-E was directly involved in these pathways. In contrast, we found clear evidence that siglec-E is able to modulate CD11b-dependent “outside-in” signaling. Siglec-E–deficient neutrophils were seen to exhibit exaggerated phosphorylation of both Syk and p38 MAP kinase following adhesion to fibrinogen. This effect was specific to CD11b ligation because there were no differences in Syk or p38 phosphorylation when cells were plated on immune complexes to ligate Fc receptors or after LPS stimulation. Furthermore, siglec-E–dependent suppression of Syk phosphorylation was selective to only one of three tyrosine residues examined, namely Y317Syk.
A number of studies have demonstrated the importance of Syk in modulating neutrophil activation events including adhesive functions (for a recent review, see Mócsai et al32 ). Using Syk-deficient neutrophils, it was shown that Syk plays an important role in adhesion strengthening in vivo via synergy with fMLP-dependent signaling.28 In human dHL-60 cells, phospho-Y323Syk (equivalent to Y317Syk in mouse) can selectively bind the p85 regulatory subunit and trigger translocation of PI3Kδ to the leading edge where it plays a role in polarization and directed movement.33 This raises the possibility that the siglec-E–dependent suppression of phospho-Y317Syk shown here modulates neutrophil migration through a similar mechanism. p38 MAP kinase has been shown to play a role in recruitment of neutrophils to the lung induced by aerosolized LPS34 and has also been implicated in P-selectin glycoprotein ligand 1 (PSGL-1) and Syk-dependent upregulation of lymphocyte function associated antigen (LFA-1) affinity in neutrophils, resulting in slow rolling on intercellular adhesion molecule-1 substrate.35 Negative regulation of p38 by siglec-E may therefore also contribute to suppression of neutrophil recruitment to the lung.
We investigated the mechanism by which siglec-E is able to suppress CD11b-dependent Syk activation. Clear evidence was obtained that sialic acid recognition was involved because the siglec-E–dependent suppression of Syk phosphorylation was specifically reversed when fibrinogen was pretreated with sialidase. These findings suggest that the siglec-E–dependent inhibition of CD11b signaling requires trans interactions between siglec-E on neutrophils and sialic acids presented on CD11b ligands. Coligation of siglec-E and CD11b with fibrinogen may be important for bringing these molecules in sufficient proximity to permit siglec-E–dependent inhibitory effects. By confocal microscopy, siglec-E was present as punctate foci at the substrate interface. Although, no precise colocalization with CD11b was observed, significant numbers of siglec-E foci were found in areas of intense CD11b staining in comparison with regions of low CD11b intensity. It is possible that the active “signaling-competent” CD11b/DAP12/FcRγ complexes36 represent a minor pool of total CD11b and that they are transient in nature.
Fibrinogen is thought to be important in promoting LPS-triggered lung neutrophil recruitment in vivo.37 However, it is likely that additional CD11b glycoprotein ligands such as intercellular adhesion molecule-138 are rapidly upregulated in vivo on LPS-activated lung capillary endothelial cells and play a role in siglec-E–dependent suppression of neutrophil recruitment. Siglec-E is a promiscuous sialic acid binding receptor and is therefore likely to bind a range of sialylated glycoproteins and glycolipids on the endothelial cell surface.39 Indeed, preliminary results have shown strong sialic acid–dependent binding of recombinant soluble forms of siglec-E to ex vivo endothelial cells (S.J.M. and P.R.C., unpublished observations, 2012). Further studies are required to investigate the expression and nature of potential siglec-E counter-receptors.
As a second line of investigation, we asked if CD11b ligation of neutrophils led to SHP-1 and SHP-2 interactions with siglec-E, which could be important in reducing tyrosine phosphorylation of Syk downstream of CD11b ligation. Interestingly, we showed that in neutrophils, siglec-E is constitutively associated with SHP-1 but not with SHP-2. This constitutive association is likely to be important in allowing siglec-E to function as a negative regulator of Syk and p38 MAP kinase and for suppression of neutrophil recruitment in the lung. It has previously been shown that Syk is a substrate for SHP-1–mediated dephosphorylation,40 but the site(s) have not been mapped. Our finding that Y317-Syk is selectively hyperphosphorylated in siglec-E–deficient neutrophils raises the possibility that siglec-E directs SHP-1 to this particular site, because the neighboring Y346Syk was not affected. Further studies are needed to investigate the full impact of phospho-Y317Syk dephosphorylation on Syk-dependent functions in neutrophils.
In a previous study of siglec-5–transfected rat basophil leukemia cells, it was shown that tyrosine phosphorylation was not required for inhibitory signaling, and this correlated with weak SHP-1 association even when the two siglec-5 ITIM tyrosine residues were mutated to alanine.41 Thus, some CD33-related siglecs appear to be unusual among ITIM-containing inhibitory receptors in not requiring Src family kinase–mediated ITIM phosphorylation to recruit SHP-1 and inhibit activation events. This mechanism may have evolved to maintain resting leukocytes in a quiescent state until activation is triggered via other activating receptors that are not influenced by siglec-E, such as Fc receptors.
A key role for SHP-1 in inflammatory responses has been demonstrated by the overexpansion and inappropriate activation of granulocyte populations in SHP-1–deficient motheaten mice.8 SHP-1–deficient neutrophils exhibit increased oxidant production, surface expression of CD18, and adhesion to protein-coated plastic, suggesting a crucial role for SHP-1 in modulating neutrophil activation.7 To date, the best-characterized targets for SHP-1 in neutrophils are Ly49Q and CEACAM1. Ly49Q has been shown to inhibit neutrophil adhesion by preventing focal-complex formation, whereas following exposure to inflammatory stimuli, Ly49Q can switch functions and promote neutrophil polarization and tissue infiltration.9 In comparison, CEACAM1 plays a key role in SHP-1–dependent expansion of bone marrow neutrophils via blocking granulocyte macrophage–CSF-signal transducer and activator of transcription 3 signaling.42 A recent study has shown that CEACAM1 can also regulate TLR-4–triggered inflammasome activation via SHP-1–dependent negative regulation of Syk activation.43 The findings of the present study demonstrate that siglec-E is another important neutrophil receptor that constitutively associates with SHP-1, selectively suppresses CD11b β2 integrin signaling, and controls early inflammatory responses in the lung. Our findings have implications for the functions of the highly related human siglec-914,44 and raise the possibility that siglec-9 could be a new therapeutic target in inflammatory lung disease.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Laura McCarthy for help with analysis of siglec-E–deficient mice, Dr Patrick Cao for help with adhesion assays and discussions, Dr Iain McMillan (Veterinary Diagnostic Services Laboratory, University of Glasgow) for histology services, and Dr Angelika Gründling (Imperial College London) for the generous gift of S aureus.
This work was supported by a Wellcome Trust Senior Fellowship WT081882.
Wellcome Trust
Authorship
Contribution: S.J.M., R.S.S., E.J.M., A.P., and H.E.R. performed experiments; J.Z. generated key reagents; and S.J.M. and P.R.C. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paul Crocker, Division of Cell Signalling and Immunology, College of Life Sciences, University of Dundee, Dundee DD1 5EH, Scotland, United Kingdom; e-mail: p.r.crocker@dundee.ac.uk.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal