Key Points
Histamine and serotonin induce, but subsequently truncate, angiogenesis via a thrombspondin-1–mediated negative feedback loop.
Abstract
Angiogenesis plays an important role in cancer and in many other human diseases. Vascular endothelial growth factor-A (VEGF-A), the best known angiogenic factor, was originally discovered as a potent vascular permeability factor (VPF), suggesting that other vascular permeabilizing agents, such as histamine and serotonin, might also have angiogenic activity. We recently demonstrated that, like VEGF-A, histamine and serotonin up-regulate the orphan nuclear receptor and transcription factor TR3 (mouse homolog Nur77) and that TR3/Nur77 is essential for their vascular permeabilizing activities. We now report that histamine and serotonin are also angiogenic factors that, at low micromolar concentrations, induce endothelial cell proliferation, migration and tube formation in vitro, and angiogenesis in vivo. All of these responses are mediated through specific histamine and serotonin receptors, are independent of VEGF-A, and are directly dependent on TR3/Nur77. Initially, the angiogenic response closely resembled that induced by VEGF-A, with generation of “mother” vessels. However, after ∼10 days, mother vessels began to regress as histamine and serotonin, unlike VEGF-A, up-regulated the potent angiogenesis inhibitor thrombospondin-1, thereby triggering a negative feedback loop. Thus, histamine and serotonin induce an angiogenic response that fits the time scale of acute inflammation.
Introduction
Histamine and serotonin (5-hydroxytryptamine [5-HT]) are biogenic amines with multiple essential functions in vivo and on cultured cells.1-5 Both have important roles in acute inflammation and are important neurotransmitters in the central nervous system. Histamine is widely expressed in mammalian tissues by neurons, mast cells and basophils, macrophages, parietal cells of the stomach, many cancer cells, etc. The activities of histamine are mediated through 4 G-protein–coupled receptors (H1-H4) that exhibit different tissue distributions.6 H1 is widely distributed and, in addition to roles in the central nervous system, mediates the roles of histamine in immediate hypersensitivity reactions.2,7 In these reactions, histamine released from mast cells or basophils has potent effects on vascular smooth muscle cells, causing cell contraction and division, and on vascular endothelial cells (EC), inducing microvascular permeability and, disputably, EC division. H2 also mediates neurotransmission but, additionally, gastric acid secretion and T-lymphocyte function. H3 serves primarily as a neurotransmitter, whereas the more recently discovered H4 is distributed in cells of hematopoietic lineage and mediates functions such as inflammatory cell chemotaxis, cytokine release, and T-lymphocyte activation.
Like histamine, serotonin has multiple functions in the central nervous system and is concentrated peripherally in enterochromaffin cells of the gastrointestinal tract, where it has a role in motility.1,3,8,9 More relevant to the present discussion, serotonin is also found in platelet dense granules and, along with histamine, in mast cell granules. On mast cell or platelet degranulation, serotonin serves as a proinflammatory mediator that increases vascular permeability and is mitogenic for smooth muscle cells and vascular EC.9-11 Serotonin also activates monocytes, preventing apoptosis and modulating cytokine and chemokine production. The activities of serotonin are mediated through 7 classes of receptors (14 different proteins), as well as independently of receptors through the serotonin transporter, which facilitates reuptake of serotonin in neuronal presynapses.1,10 EC are reported to express several different serotonin receptors including 5-HT1, 5-HT2, and 5-HT4.12,13
In addition to the multiple and varied functions listed above, there have been claims that histamine and serotonin have roles in angiogenesis. As early as 1969, Zauberman et al14 reported that both amines induced new blood vessel formation when introduced into the rabbit cornea. Many studies have implicated histamine in pathologic angiogenesis,15-17 but mechanistic studies to date have showed this action to be indirect through upregulation of VEGF-A expression.18 Less is known about a role for serotonin in angiogenesis, though serotonin does affect EC signaling in culture,19 and serotonin-deficient (tryptophan hydroxylase 1 null) mice exhibit decreased tumor angiogenesis.8 Furthermore, Jackson et al20 reported a role for serotonin in the angiogenesis induced by metastatic carcinoids.
Vascular endothelial growth factor-A (VEGF-A) is the classic tumor angiogenesis factor but was originally discovered as a potent vascular permeability factor (VPF).21 Recent studies have done much to elucidate the steps and mechanisms by which VEGF-A induces angiogenesis and enhances permeability.22 Many signaling pathways have been implicated, but most, if not all, begin with activation of VEGF-A receptor 2 (VEGFR-2/KDR/Flk-1).23 Activation of VEGFR-2, in turn, leads to the upregulation of the orphan nuclear receptor and transcription factor TR3 (human)/Nur77 (mouse), and TR3/Nur77 mediates most, if not all, of the angiogenic and vascular permeabilizing activities of VEGF-A.24,25 We recently reported that histamine and serotonin also up-regulate TR3/Nur77 and that TR3/Nur77 is essential for their vascular permeabilizing activities.25 This finding was unexpected in that histamine and serotonin act through G-coupled receptors that are unrelated to VEGFR-2.
Together, these findings prompted us to investigate whether histamine and serotonin induced angiogenesis and, if so, by what mechanisms. Here, we report that both histamine and serotonin, acting through their specific receptors and independent of VEGF-A and VEGFR-2, induce proliferation of human umbilical vein EC (HUVEC), migration and tube formation in vitro, and angiogenesis in vivo. Moreover, like VEGF-A, these actions are mediated through TR3/Nur77. As in response to VEGF-A, the first new blood vessels formed in vivo were “mother” vessels (MV), greatly enlarged, highly permeable, pericyte-poor sinusoids. Histamine or serotonin, acting on EC in culture or on tissue sites in vivo, initially reduced expression of thrombspondin-1 (TSP-1), a potent angiogenesis inhibitor,26 thus favoring angiogenesis. Subsequently, however, histamine and serotonin, unlike VEGF-A, up-regulated the TSP-1 promoter and restored TSP-1 levels to normal. This triggered a negative feedback loop that caused vascular regression and thus imposed a temporal limit on the angiogenic response induced by histamine and serotonin.
Materials and Methods
Materials
VEGF-A165 (human) and VEGF-A164 (mouse) were purchased from R&D Systems (Minneapolis, MN). Histamine (2-(4-Imidazolyl)ethylamine); serotonin (5-HT); the VEGFR-2 (KDR) receptor inhibitor SU1498; the H1 histamine receptor antagonist, mepyramine (N-(4-Methoxyphenyl)methyl-N′,N′-dimethyl-N-(2-pyridinyl)-1,2-ethanediamine); the H2 histamine receptor antagonist, Zolantidine (N-[3-[3-(1-piperidinylmethyl)phenoxy]propyl]-2-benzothiazolamine); the H4 histamine receptor antagonist, JNJ777120; the serotonin (5HT) receptor 1,6,7 antagonist, M149 (1-[10,11-dihydro-8-(methylthio)dibenzo[b,f] thiepin-10-yl]-4-methylpiperazine mesylate); the serotonin (5HT) receptor 2 antagonist, S006 (3-(2-[4-(4-fluorobenzoyl)-1-piperidinyl]ethyl)-2,4(H1,H3)-quinazolinedione (+)-tartrate); and the serotonin (5HT) receptor 3 antagonist, T113 (3-tropanylindole-3-carboxylate methiodide) were purchased from Sigma (St. Louis, MO). Antibodies against TR3/Nur77 and mouse CD31 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). An antibody prepared in chickens against mouse TSP-1 was used for immunoblots, as described previously.27
Cell culture and assays
HUVEC (Lonza, Walkersville, MD) were cultured and transduced with retroviruses carrying various genes, as previously described.28 At 80% confluence, HUVEC were incubated in 0.1% fetal bovine serum–containing endothelial basal medium for 24 hours and then were treated, in all assays described below, with VEGF-A165 (10 ng/mL), histamine (10 μM), or serotonin (1 μM), as previously described.28 In all of the in vitro assays that follow, receptor antagonists (40 μg/mL of an H1 antagonist, 10 μM of an H2 antagonist, 3 μM of an H4 antagonist, 0.25 μM of a serotonin (5HT) receptor 1,6,7 antagonist, 10 μg/mL of a serotonin (5HT) receptor 2 antagonist, and 3 μg/mL of a serotonin (5HT) receptor 3 antagonist) were added 10 minutes before addition of VEGF-A165, histamine, or serotonin, whereas 3TSR (50 nM) was added 30 minutes before.
Proliferation assay
HUVEC (2 × 103) were seeded in 24-well plates. After 2 days, cells were serum-starved (0.1% serum) for 24 hours, pretreated with antagonists or inhibitors, and then stimulated with VEGF-A165, histamine, or serotonin for 20 hours. Thereafter, 1 µCi of 3H-thymidine was added to each well, and 4 hours later cells were washed with cold phosphate-buffered saline (PBS) 3 times, fixed with 100% cold methanol for 15 minutes at 4°C, precipitated with 10% cold trichloracetic acid for 15 minutes at 4°C, washed with water 3 times, and lysed with 200 µL 0.1N NaOH for 30 minutes at room temperature for scintillation counting.
Transwell migration assay
The serum-starved HUVEC (as described above) were washed 2 times with PBS and were incubated for 20 minutes at 37°C with 4 mL of collagenase solution (0.2 mg/mL of collagenase (Sigma), 0.2 mg/mL soybean trypsin inhibitor, 1 mg/mL of bovine serum albumin, 2 mM of EDTA in PBS). Cells were detached by gentle scraping and were centrifuged at 1100 rpm for 3 minutes, washed 2 times with endothelial basal medium containing 1% bovine serum albumin, seeded (1 × 105 cells per well) on transwell filters coated with Pure Col (30 µg/mL; Advanced BioMatrix, San Diego, CA), and inserted in 24-well plate wells containing 1 mL of the same medium. Cells were incubated at 37°C for 1 hour to allow cell adhesion, after which antagonists or inhibitors were added followed by addition of VEGF-A165, histamine, or serotonin to the bottom well. After 2-hour incubation, cells remaining on the upper surface of the transwell filter membrane were wiped off with a cotton tip, and the membranes were incubated with CyQUANT (Molecular Probes, Eugene, OR) DNA stain overnight at 4°C. After warming to room temperature, stained cells were counted in a spectrofluorometer (SpectraFluor; TECAN) using Δ Soft 3 software. A standard curve was prepared using cells seeded over a range of 3 × 103 to 1 × 105 cells per well in a 96-well plate, and these cells were incubated and stained as described for stimulated cells.
Monolayer migration (wound-healing) assay
The HUVEC wound-healing assay was carried out as described.29 Confluent HUVEC on 24-well plates were subjected to serum starvation, as described above. The monolayer was then wounded with a single pass of a 200-µL pipette tip; washed with PBS; photographed with an Axiovert 35 microscope (Zeiss, Oberkochen, Germany) and a DFC350FX camera (Leica, Allendale, NJ) with Leica FireCam software; pretreated with or without antagonists or inhibitors; and then incubated for 16 hours in the presence or absence of VEGF-A165, histamine, or serotonin. Thereafter, the monolayer was washed with PBS and photographed (microscope). Data are expressed as the number of cells migrating into the wounded area (20 views from 3 independent experiments).
EC tube formation assay
Serum-starved HUVEC were seeded at a density of 1 × 105 cells in 12-well plates that were precoated with 0.5 mL of growth-factor–reduced Matrigel; pretreated with or without inhibitors; incubated for 6 hours in the presence or absence of VEGF-A165, histamine, or serotonin; washed with PBS; and photographed. Pictures were taken with the Axiovert 35 microscope and the DFC350FX camera with Leica FireCam software. Figures were analyzed with Image J software, and total tube lengths were calculated in 20 views from each of the 3 independent experiments (20× microscopic fields).
Animals
Female, 4-to 5-week Nu/Nu mice were purchased from the National Institutes of Health and C57Bl/6 wild-type mice from Charles River Laboratories. Nur77−/− mice were obtained originally from Dr. Jeffrey Milbrandt (Washington University School of Medicine, St. Louis, MO)30 and have subsequently been bred in house. All animal experiments were performed in compliance with the Beth Israel Deaconess Medical Center’s Institutional Animal Care and Use Committee.
Adeno- and adeno-associated viruses
An adenovirus expressing VPF/VEGF (VEGF-A164, 1 × 107 pfu in 10 μL) was injected to induce angiogenesis in the ears of Nu/Nu mice, as previously described.31 An adeno-associated virus (AAV1) expressing 3TSR was generated to test the effects of TSP-1 on histamine- and serotonin-induced angiogenesis.32
Angiogenesis induced with histamine- and serotonin-releasing pellets
Flank hair was clipped as necessary 1 day before the experiments. Nude, C57Bl/6 wild-type and Nur77−/− mice were implanted subcutaneously with control pellets or pellets that released histamine or serotonin continuously for 21 days (Innovative Research of America, Sarasota, FL). At indicated times, tissues were dissected and photographed with a Wild M400 Karema Umrustkit (ProMicron, Kirchheim, Neckar), SPOT 14.2-color Mosaic camera (Spot Imaging Solutions, Sterling Heights, MI) and Spot software, or were homogenized in ice-cold T-PER tissue protein extraction reagent (Pierce Biotechnology, Rockford, IL) containing phenylmethylsulfonyl fluoride (1mM), Na3VO4 (1mM), EGTA (1 mM), leupeptin (1 mM), aprotinin (1 mg/mL), and pepstatin A (2 ng/mL). In some experiments, histamine or serotonin receptor antagonists or a VEGF-A165 receptor inhibitor were administered as well.
Histology and immunohistochemistry
Tissues were fixed and prepared for Giemsa-stained, 1-μm-thick Epon sections, as previously described.31 For immunohistochemistry, tissues were fixed in 4% paraformaldehyde and embedded in optimal cutting temperature compound.24 Frozen sections were treated with 0.3% H2O2 for 5 minutes and were subjected to antigen retrieval with 100 mM of Tris-HCl in boiling water for 15 minutes. Sections were blocked with PBS-5% donkey serum at room temperature for 1 hour, incubated with rat anti-mCD31 (1:100 dilution, BD Biosciences, San Jose, CA) in PBS-5% donkey serum (200 µL) at 4°C overnight, rinsed with PBS 3 times, incubated for 45 minutes with donkey anti-rat IgG in PBS-5% donkey serum (200 µL) at room temperature for 1 hour, rinsed with PBS 3 times, and stained with the Vectastain ABC Kit (Vector Laboratories, Burlingame, CA). Pictures (RGB) were taken with an Axio Imager A1 Microscope (Zeiss), AxioCam (Zeiss), and Axio Vision Rel 4.8 software. Vascular density was measured with Image J software based on counts of ten 20× microscopic fields.
Vascular permeability assays
Flank hair skin was clipped and depilated 1 day before the mice were anesthetized with Avertin (tribromoethanol, 200 mg/kg), injected intravenously with 0.2 mL of Evans blue dye (0.5 mg/mL in saline), and injected intradermally in flank skin with pellet extracts or Hanks balanced salt solution (HBSS). Flank injection sites were photographed 30 minutes later. Dye was extracted with formamide and was quantified in a spectrophotometer at 620 nm in a Thermo Max microplate reader (Molecular Devices, Menlo Park, CA) using Softmax 881 software.
TSP-1 promoter activity assay
HUVEC (1 × 105 cells/well) were cultured in 12-well plates for 24 hours and were transfected with TSP-1 reporter plasmids and a control luciferase plasmid (pRL-tk) using a FuGENE-6 transfection reagent (Roche Diagnostics, Indianapolis, IN). Cells were then washed 2 times with PBS. Two microliters of FuGENE was added to 50 μL of OPTI-MEM1 medium and was incubated for 5 minutes. TSP-1 promoter plasmid (0.5 μg) and pRL-tk (0.2 μg) were added to the mixture, incubated for 15 minutes, and then were added to cells in 300 μL of culture medium. Twenty-four hours after transfection, cells were stimulated with histamine, serotonin, or VEGF-A165 for indicated times, washed with PBS, and lysed with 100 μL of passive lysis buffer (Dual-luciferase Reporter Assay System; Promega, Madison, WI) at room temperature. Luciferase activity was assayed and was normalized to equal internal control luciferase activity according to the manufacturer’s protocol.
Statistics
Statistics were performed with the Tukey-Kramer or Kruskal-Wallis multiple comparison tests, or with the Student t test, as indicated, using InStat software (GraphPad, San Diego, CA).
Results
Histamine and serotonin induce HUVEC proliferation, migration, and tube formation in vitro
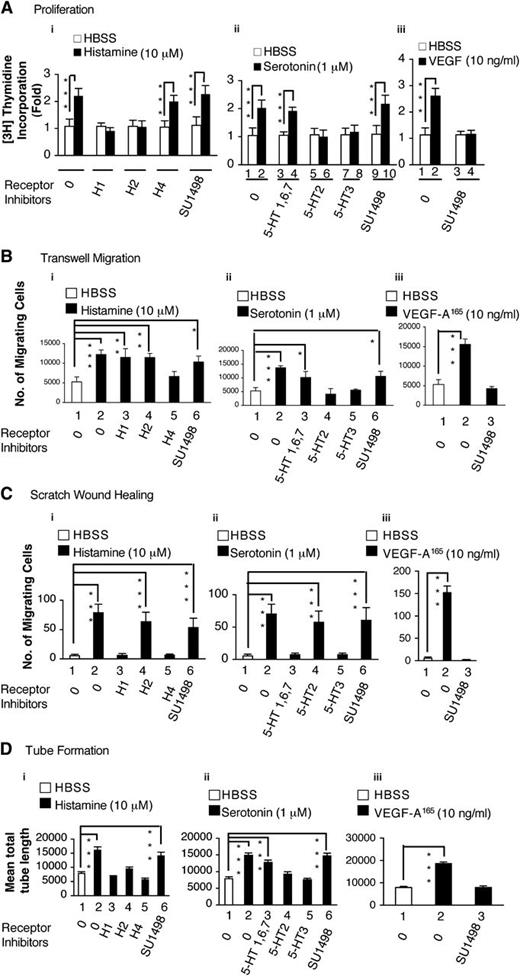
We used several different assays to assess the ability of histamine and serotonin to modify the behavior of cultured EC. As shown in Figure 1, both amines significantly stimulated HUVEC incorporation of 3H thymidine, a measure of cell proliferation (Figure 1Ai-ii); HUVEC migration in transwell chambers (Figure 1Bi-ii); HUVEC migration in the scratch monolayer wound-healing assay (Figure 1Ci-ii; supplemental Figure 1A); and HUVEC tube formation on Matrigel (Figure 1Di-ii; supplemental Figure 1B). In all of these assays, histamine and serotonin performed about as well as VEGF-A165 (Figure 1A-Diii). SU1489, a selective inhibitor of VEGFR-2, did not inhibit the activities of either histamine or serotonin in any of these assays, though it potently inhibited the activity of VEGF-A165 (Figure 1A-Diii). On the other hand, selective inhibitors of the various histamine and serotonin receptors effectively inhibited histamine and serotonin functions in each of these assays, though different receptor inhibitors were active in different assays (Figure 1, Table 1).
Actions of histamine and serotonin on cultured EC. (A) H3 thymidine incorporation. (B,C) Transwell and scratch monolayer migration. (D) Tube formation. Serum-starved HUVEC, with or without pretreatment with indicated inhibitors or antagonists, were stimulated with histamine (10 μM), serotonin (1 μM), or VEGF-A165 (10 ng/mL), as described in “Methods.” Results are summarized in Table 1. (A) 3H thymidine incorporation assay (n = 4; data are representative of 3 independent experiments). (B) Transwell migration (n = 4; data are representative of 3 independent experiments). (C) Scratch monolayer migration (total n = 20 from three independent experiments). (D) Tube formation (n = 20 from 3 independent experiments). *P < .05; **P < .01; ***P < .001. Tukey-Kramer multiple comparisons test (A, B, D); Kruskal-Wallis test with Dunn multiple comparison (C).
Actions of histamine and serotonin on cultured EC. (A) H3 thymidine incorporation. (B,C) Transwell and scratch monolayer migration. (D) Tube formation. Serum-starved HUVEC, with or without pretreatment with indicated inhibitors or antagonists, were stimulated with histamine (10 μM), serotonin (1 μM), or VEGF-A165 (10 ng/mL), as described in “Methods.” Results are summarized in Table 1. (A) 3H thymidine incorporation assay (n = 4; data are representative of 3 independent experiments). (B) Transwell migration (n = 4; data are representative of 3 independent experiments). (C) Scratch monolayer migration (total n = 20 from three independent experiments). (D) Tube formation (n = 20 from 3 independent experiments). *P < .05; **P < .01; ***P < .001. Tukey-Kramer multiple comparisons test (A, B, D); Kruskal-Wallis test with Dunn multiple comparison (C).
Summary of effects of various antagonists on histamine, serotonin, or VEGF-A stimulation in different in vitro and in vivo angiogenic assays*
| . | Histamine receptor inhibitors . | Serotonin receptor inhibitors . | ||
|---|---|---|---|---|
| Assay . | Inhibition . | No inhibition . | Inhibition . | No inhibition . |
| 3H thymidine incorporation | H1; H2 | H4 | 5-HT2; 5-HT3 | 5-HT1, 6,7 |
| Transwell migration | H4 | H1; H2 | 5-HT2; 5-HT3 | 5-HT1, 6,7 |
| Wound-healing migration | H1; H4 | H2 | 5-HT1, 6, 7; 5-HT3 | 5-HT2 |
| Tube formation | H1; H2; H4 | - - | 5-HT2; 5-HT3 | 5-HT1, 6,7 |
| Angiogenesis in vivo | H1; H2 | H4 | 5-HT2; 5-HT3 | 5-HT1, 6,7 |
| . | Histamine receptor inhibitors . | Serotonin receptor inhibitors . | ||
|---|---|---|---|---|
| Assay . | Inhibition . | No inhibition . | Inhibition . | No inhibition . |
| 3H thymidine incorporation | H1; H2 | H4 | 5-HT2; 5-HT3 | 5-HT1, 6,7 |
| Transwell migration | H4 | H1; H2 | 5-HT2; 5-HT3 | 5-HT1, 6,7 |
| Wound-healing migration | H1; H4 | H2 | 5-HT1, 6, 7; 5-HT3 | 5-HT2 |
| Tube formation | H1; H2; H4 | - - | 5-HT2; 5-HT3 | 5-HT1, 6,7 |
| Angiogenesis in vivo | H1; H2 | H4 | 5-HT2; 5-HT3 | 5-HT1, 6,7 |
SU1489, an inhibitor of VEGFR-2, fully inhibited VEGF-A stimulation, but not that of histamine or serotonin, in all of the above assays.
Histamine and serotonin slow-release pellets induce angiogenesis in vivo
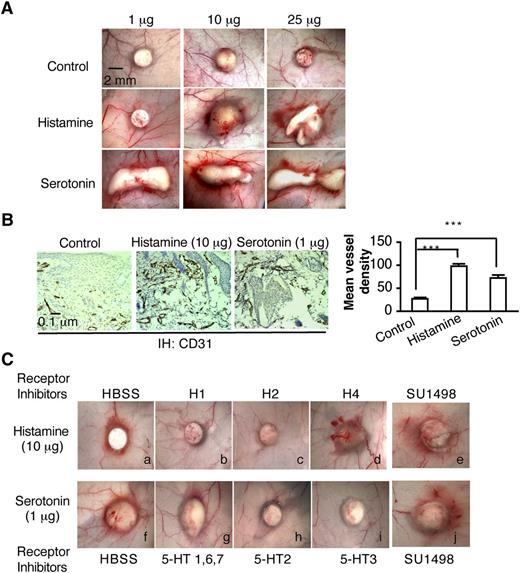
Biodegradable, slow-release pellets containing varying amounts of histamine or serotonin were implanted into the subcutaneous space of Nu/Nu mice, where they induced angiogenesis in a dose-dependent manner (Figure 2A-B). New blood vessel formation was apparent as early as 3 days, whereas control pellets induced no significant angiogenesis. On the basis of these preliminary experiments, we chose pellets containing 10 μg of histamine and 1μg of serotonin for further study. These pellets are reported to release histamine and serotonin continuously and at constant rates (ie, 20 and 2 ng/h, respectively) for 21 days and therefore are expected to achieve tissue concentrations of these amines that are well within the pathophysiologic range.33,34 H1 and H2 antagonists, but not the H4 antagonist, greatly inhibited histamine pellet–induced angiogenesis (Figure 2Ca-d). Antagonists of the 5-HT2 and 5-HT3 serotonin receptors inhibited serotonin-induced angiogenesis, whereas an antagonist of the 5-HT1, 6, and 7 serotonin receptors, had no effect (Figure 2Cf-i). Again, the VEGFR-2 kinase inhibitor SU1498, which potently inhibits VEGF-A165–induced angiogenesis and microvascular hyperpermeability,24,35 had no effect on histamine- or serotonin-induced angiogenesis and microvessel permeability (Figure 2Ce,j). Together, these data demonstrate that histamine and serotonin induce angiogenesis directly through their specific receptors.
Histamine and serotonin induce angiogenesis in vivo. (A) Female, 4-to 5-week Nu/Nu mice were implanted subcutaneously with control pellets or pellets that contained indicated amounts of histamine or serotonin. Tissues were dissected and photographed 3 days later. (B) Tissues adjacent to pellet implant sites were immunostained with an antibody against CD31. Mean vessel density was calculated with Image J software as described in “Methods” (n = 10 from 4 animals per group). ***P < .001, Student t test. (C) Effect of various histamine and serotonin receptor antagonists on angiogenesis induced in Nu/Nu mice by histamine- or serotonin-releasing pellets. Inhibitors (1mg/kg) were injected intraperitoneally daily, beginning when the pellets were implanted; in addition, a single injection of inhibitor (1 micromole in 50 μl or HBSS as control) was administered intradermally daily directly over the pellet implant site. Tissues were harvested and photographed on day 6. Histamine-induced angiogenesis was strongly inhibited by 1H and H2 antagonists but not by an antagonist of H4. Serotonin-induced angiogenesis was strongly inhibited by an antagonist of serotonin receptor types 2 and 3, but not by antagonists of types 1, 6, and 7. SU1498, an inhibitor of VEGFR-2, had no effect on angiogenesis induced by either histamine or serotonin. Data are representative of 8 mice in each group.
Histamine and serotonin induce angiogenesis in vivo. (A) Female, 4-to 5-week Nu/Nu mice were implanted subcutaneously with control pellets or pellets that contained indicated amounts of histamine or serotonin. Tissues were dissected and photographed 3 days later. (B) Tissues adjacent to pellet implant sites were immunostained with an antibody against CD31. Mean vessel density was calculated with Image J software as described in “Methods” (n = 10 from 4 animals per group). ***P < .001, Student t test. (C) Effect of various histamine and serotonin receptor antagonists on angiogenesis induced in Nu/Nu mice by histamine- or serotonin-releasing pellets. Inhibitors (1mg/kg) were injected intraperitoneally daily, beginning when the pellets were implanted; in addition, a single injection of inhibitor (1 micromole in 50 μl or HBSS as control) was administered intradermally daily directly over the pellet implant site. Tissues were harvested and photographed on day 6. Histamine-induced angiogenesis was strongly inhibited by 1H and H2 antagonists but not by an antagonist of H4. Serotonin-induced angiogenesis was strongly inhibited by an antagonist of serotonin receptor types 2 and 3, but not by antagonists of types 1, 6, and 7. SU1498, an inhibitor of VEGFR-2, had no effect on angiogenesis induced by either histamine or serotonin. Data are representative of 8 mice in each group.
Histamine- and serotonin-induced angiogenesis is dependent on TR3/Nur77
We recently reported that the orphan nuclear receptor TR3 (mouse analog Nur77) is required for the vascular permeabilizing activities of VEGF-A, histamine, and serotonin,25 in addition to its requirement for VEGF-A–induced HUVEC proliferation, tube formation, and angiogenesis in Matrigel.24 Therefore, we considered the possibility that histamine and serotonin might also require TR3/Nur77 for their angiogenic activities. Initially, we took an antisense approach. We showed that transduction of HUVEC with TR3 antisense cDNA reduced TR3 expression by more than 90%, whereas transduction with LacZ had no effect on TR3 expression.24 Furthermore, TR3 antisense DNA almost completely inhibited VEGF-A165–stimulated HUVEC proliferation in vitro and Matrigel angiogenesis in vivo, whereas transduction with LacZ had no effect.24 We have now extended these findings to histamine- and serotonin-induced stimulation of cultured EC. HUVEC transduced with TR3 antisense cDNA exhibited greatly reduced incorporation of 3H thymidine; migration in transwell chambers and in scratch wound-healing assays; and tube formation in response to VEGF-A165, histamine, or serotonin (Figure 3A-D, supplemental Figure 2A-B). In addition, almost no angiogenesis developed when histamine or serotonin pellets were implanted in Nur77−/− mice (Figure 3E).
Histamine- and serotonin-induced angiogenesis require TR3/Nur77. (A-D) Effects of TR3-antisense vs LacZ, as control, on histamine-, serotonin- and VEGF-A165–induced 3H thymidine incorporation (n = 4; experiments repeated 3 times), transwell migration (n = 4; experiments repeated 3 times), scratch wound-healing assay migration (n = 20; 3 independent experiments), and tube formation (n = 4; experiments repeated 3 times). Assays were performed as in Figure 1A-D. **P < .01 and ***P < .001, Student t test. (E) Histamine- and serotonin- releasing pellets were harvested 6 days after implantation in Nur77−/− vs Nur77+/+ wild-type mice. Angiogenesis with MV (red arrows) developed in Nur77+/+ but not in Nu77−/− mice. Data are representative of experiments performed on 8 mice in each group.
Histamine- and serotonin-induced angiogenesis require TR3/Nur77. (A-D) Effects of TR3-antisense vs LacZ, as control, on histamine-, serotonin- and VEGF-A165–induced 3H thymidine incorporation (n = 4; experiments repeated 3 times), transwell migration (n = 4; experiments repeated 3 times), scratch wound-healing assay migration (n = 20; 3 independent experiments), and tube formation (n = 4; experiments repeated 3 times). Assays were performed as in Figure 1A-D. **P < .01 and ***P < .001, Student t test. (E) Histamine- and serotonin- releasing pellets were harvested 6 days after implantation in Nur77−/− vs Nur77+/+ wild-type mice. Angiogenesis with MV (red arrows) developed in Nur77+/+ but not in Nu77−/− mice. Data are representative of experiments performed on 8 mice in each group.
The angiogenic responses induced by histamine and serotonin initially resemble that induced by VEGF-A165 but are transitory
The angiogenic responses induced by histamine and serotonin progressed through ∼day 10 but then began to regress so that, by 21 days, very few of the newly formed blood vessels remained (Figure 4A). This finding is in contrast to that in tumors in which angiogenesis, induced primarily by the overexpression of VEGF-A, continues progressively. A similar, continuously progressive angiogenic response can be induced in nude mice by an adenoviral vector engineered to express VEGF-A164 (Ad-VEGF-A164).22 Ad-VEGF-A164 induces a succession of new, abnormal blood vessels that derive from preexisting venules and capillaries. MV form first and, with time, evolve into several types of “daughter” vessels that persist for many months.22
The angiogenic responses induced by histamine and serotonin initially resembled that induced by VEGF-A165 but were transient. (A) Macroscopic appearance of progression and subsequent regression of angiogenesis with time in Nu/Nu mice implanted with histamine- and serotonin-containing pellets vs control pellets; (Ba-c) Angiogenic responses induced by histamine at 1, 5, and 15 days after implantation, respectively. Note characteristic MV at 1 day, vessel remodeling at 5 days, and striking vessel regression at 15 days. (Bd-f) Angiogenic responses induced by serotonin at 3, 7, and 21 days after implantation, respectively. Prominent MV are present at 3 days, which undergo remodeling thereafter (7 days) and regression (21 days). One-micron-thick, Giemsa-stained Epon sections.31 Magnification bars, 50 μm. Data are representative of 4 mice in each group. (C) Tissues adjacent to pellet implant sites were immunostained with an antibody against CD31. Mean vessel density was calculated with Image J software (ten 20× fields, 4 animals per group). ***P < .001; **P < .01; *P < .05, Student t test.
The angiogenic responses induced by histamine and serotonin initially resembled that induced by VEGF-A165 but were transient. (A) Macroscopic appearance of progression and subsequent regression of angiogenesis with time in Nu/Nu mice implanted with histamine- and serotonin-containing pellets vs control pellets; (Ba-c) Angiogenic responses induced by histamine at 1, 5, and 15 days after implantation, respectively. Note characteristic MV at 1 day, vessel remodeling at 5 days, and striking vessel regression at 15 days. (Bd-f) Angiogenic responses induced by serotonin at 3, 7, and 21 days after implantation, respectively. Prominent MV are present at 3 days, which undergo remodeling thereafter (7 days) and regression (21 days). One-micron-thick, Giemsa-stained Epon sections.31 Magnification bars, 50 μm. Data are representative of 4 mice in each group. (C) Tissues adjacent to pellet implant sites were immunostained with an antibody against CD31. Mean vessel density was calculated with Image J software (ten 20× fields, 4 animals per group). ***P < .001; **P < .01; *P < .05, Student t test.
The histologic features of the angiogenic response induced by histamine and serotonin pellets in both nude and wild-type C57Bl/6 mice were, at first, very similar to those induced by Ad-VEGF-A164.22 Large numbers of MV formed initially, reaching a maximum at ∼10 days (Figure 4Ba,b,d,e). Thereafter, however, as predicted by the macroscopic appearance (Figure 4A), MV regressed, and only small numbers of slightly enlarged capillaries remained at 15 and 21 days (Figure 4Bc,f). Vascular density counts quantified these findings (Figure 4C).
To be certain that the regression of angiogenesis after day 10 was not attributable to a failure of histamine or serotonin content or release, we recovered pellets from mice at 14 and 21 days after implantation and suspended them in 100 μl of HBSS overnight to extract histamine or serotonin. Similar pellets that had not been implanted were extracted in the same way and served as positive controls. We then tested these extracts for activity in the Miles vascular permeability assay. We found that substantial histamine and serotonin remained in the pellets and were readily extracted at both 14 and 21 days after implantation (supplemental Figure 3).
Histamine and serotonin affect TSP-1 expression
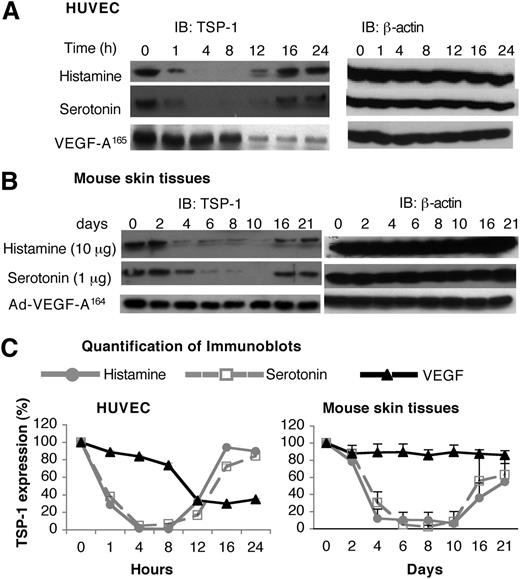
Unlike the angiogenic response induced by Ad-VEGF-A164, the response induced by histamine- and serotonin-releasing pellets was transitory (Figure 4), despite continuing release of histamine and serotonin (supplemental Figure 3). Because angiogenesis is controlled by a balance of pro- and antiangiogenic factors, we considered the possibility that histamine and serotonin might also affect the activity of an angiogenic inhibitor, such as TSP-1. Testing this possibility, we found that both histamine and serotonin induced a biphasic response in TSP-1 expression in HUVEC. Initially (1-12 h), histamine and serotonin induced a profound reduction in TSP-1 expression, but, at later times, TSP-1 levels returned to near-normal levels (Figure 5A,C). VEGF-A165 also down-regulated TSP-1 expression but with slower kinetics; also, TSP-1 levels did not return to normal but remained at reduced plateau levels (Figure 5A,C).
Effects of histamine and serotonin on TSP-1 expression. (A) Serum-starved HUVEC were stimulated with 10 μM of histamine, 1 μM of serotonin, or 10 ng/mL of VEGF-A165 for indicated times. Cell extracts were immunoblotted with a polyclonal chicken antibody specific for mouse TSP-1 (left panel). Membranes were stripped and reprobed with an antibody against β-actin to confirm equal protein loading (right panel). Data are representative of 3 separate experiments. (B) Nu/Nu mice were implanted subcutaneously with pellets containing 10 μg of histamine or 1 μg of serotonin, or were injected intradermally with 1× 107 pfu Ad-VEGF-A164. At indicated times, tissue adjacent to pellets was collected and extracted, and immunoblots were prepared using the same anti-mouse TSP-1 antibody used in (A). Data are representative of 4 separate experiments. (C) Quantification of immunoblots from 3 independent HUVEC experiments and 4 separate in vivo experiments with Image J software. Data are presented as percent change (mean ± standard error) from time zero and were analyzed with the Kruskal-Wallis test. Regarding the HUVEC data, TSP-1 levels were significantly depressed from 4 to 8 hours, but not thereafter, in the case of both histamine and serotonin treatments, whereas TSP-1 was not significantly depressed in response to VEGF until 12 hours. Regarding the histamine and serotonin in vivo data (B), TSP-1 was significantly reduced from 4 to 8 days, corresponding to the period of angiogenesis; thereafter, TSP-1 levels rebounded, correlating with angiogenesis regression (Figure 4). Ad-VEGF-A164 did not lead to reduced TSP-1 expression at any time point.
Effects of histamine and serotonin on TSP-1 expression. (A) Serum-starved HUVEC were stimulated with 10 μM of histamine, 1 μM of serotonin, or 10 ng/mL of VEGF-A165 for indicated times. Cell extracts were immunoblotted with a polyclonal chicken antibody specific for mouse TSP-1 (left panel). Membranes were stripped and reprobed with an antibody against β-actin to confirm equal protein loading (right panel). Data are representative of 3 separate experiments. (B) Nu/Nu mice were implanted subcutaneously with pellets containing 10 μg of histamine or 1 μg of serotonin, or were injected intradermally with 1× 107 pfu Ad-VEGF-A164. At indicated times, tissue adjacent to pellets was collected and extracted, and immunoblots were prepared using the same anti-mouse TSP-1 antibody used in (A). Data are representative of 4 separate experiments. (C) Quantification of immunoblots from 3 independent HUVEC experiments and 4 separate in vivo experiments with Image J software. Data are presented as percent change (mean ± standard error) from time zero and were analyzed with the Kruskal-Wallis test. Regarding the HUVEC data, TSP-1 levels were significantly depressed from 4 to 8 hours, but not thereafter, in the case of both histamine and serotonin treatments, whereas TSP-1 was not significantly depressed in response to VEGF until 12 hours. Regarding the histamine and serotonin in vivo data (B), TSP-1 was significantly reduced from 4 to 8 days, corresponding to the period of angiogenesis; thereafter, TSP-1 levels rebounded, correlating with angiogenesis regression (Figure 4). Ad-VEGF-A164 did not lead to reduced TSP-1 expression at any time point.
We also measured levels of TSP-1 in tissue extracts harvested from sites immediately adjacent to histamine and serotonin pellet implants and, for comparison, extracts prepared from skin sites of Ad-VEGF-A164 injection (Figure 5B,C). TSP-1 protein levels decreased by 88% to 93% and by 70% to 98% at histamine and serotonin injection sites, respectively, for 4 to 10 days when angiogenesis was developing; levels then rebounded in temporal accord with angiogenesis regression (Figure 4). In contrast, TSP-1 levels did not change significantly at Ad-VEGF-A164–injected skin sites during 4 to 21 days.
3TSR recombinant protein inhibits the histamine- and serotonin-induced responses of cultured HUVEC and angiogenesis in vivo
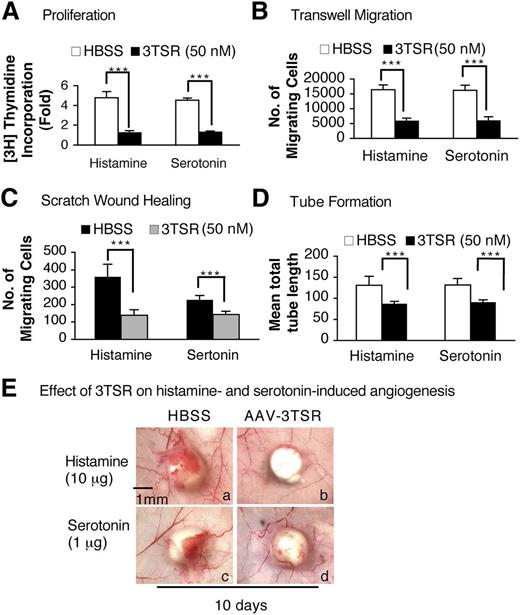
The antiangiogenic activity of TSP-1 is largely mediated by that portion of the molecule referred to as type 1 repeats, and one of us (J.L.) has prepared a recombinant protein, designated 3TSR, that contains all 3 of the type 1 repeats and that mimics the antiangiogenic action of TSP-1.36 Therefore, we tested whether 3TSR could inhibit histamine or serotonin responses on cultured EC. Serum-starved HUVEC were pretreated with 50 nM of 3TSR for 30 minutes and then were stimulated with histamine (10 μM) or serotonin (1 μM) to evaluate cell proliferation, migration, and tube formation. 3TSR completely inhibited all of these effects (Figure 6A-D, supplemental Figure 4A-B).
Modulation of histamine- and serotonin-induced EC proliferation and angiogenesis by 3TSR peptide. (A-D) Histamine- and serotonin-induced 3H thymidine incorporation, transwell and scratch assay migration, and tube formation on serum-starved HUVEC that had been pretreated for 10 minutes with 50 nM of 3TSR peptide (n = 4; experiments were repeated 3 times, A, B, and D. In C, n = 20 from 3 independent experiments). ***P < .001 vs control, Student t test. (E) Nu/Nu mice received intradermal injections of 1 × 1011 pfu of AAV-TSR in 100 μl of HBSS or HBSS alone. Two weeks later, histamine or serotonin pellets were implanted immediately beneath the AAV-TSR (b,d) or HBSS (a,c) injection sites, and tissues were collected 10 days later. Data are representative of experiments performed on 8 mice per group.
Modulation of histamine- and serotonin-induced EC proliferation and angiogenesis by 3TSR peptide. (A-D) Histamine- and serotonin-induced 3H thymidine incorporation, transwell and scratch assay migration, and tube formation on serum-starved HUVEC that had been pretreated for 10 minutes with 50 nM of 3TSR peptide (n = 4; experiments were repeated 3 times, A, B, and D. In C, n = 20 from 3 independent experiments). ***P < .001 vs control, Student t test. (E) Nu/Nu mice received intradermal injections of 1 × 1011 pfu of AAV-TSR in 100 μl of HBSS or HBSS alone. Two weeks later, histamine or serotonin pellets were implanted immediately beneath the AAV-TSR (b,d) or HBSS (a,c) injection sites, and tissues were collected 10 days later. Data are representative of experiments performed on 8 mice per group.
We then asked whether the 3TSR recombinant protein could inhibit histamine- or serotonin-induced angiogenesis in vivo. An AAV expressing the 3TSR recombinant protein (1 × 1011 pfu) was injected into the flank skin of Nu/Nu mice and the site marked with indelible ink. Two weeks later, histamine or serotonin pellets were implanted subcutaneously immediately beneath the AAV-injected sites, and tissues were collected 10 days later. As shown in Figure 6E, histamine- and serotonin-induced angiogenesis was largely inhibited in the AAV-3TSR–pretreated mice.
Histamine and serotonin regulate TSP-1 promoter activity but not directly through TR3
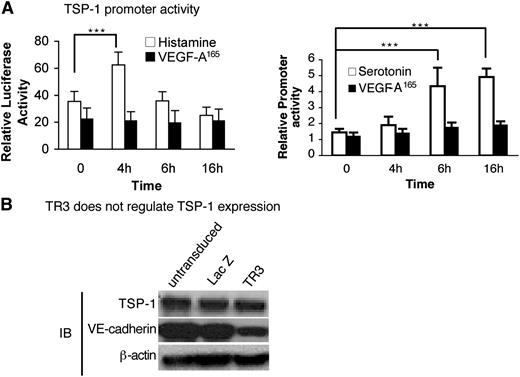
We next investigated whether histamine or serotonin acted on the TSP-1 promoter. Serum-starved HUVEC were transfected with a TSP-1 promoter-luciferase reporter or control luciferase vector and were then stimulated with histamine, serotonin, or VEGF-A165. Histamine up-regulated TSP-1 promoter activity significantly at 4 hours, and serotonin did so at both 6 and 16 hours (Figure 7A), timing that correlates well with the return of TSP-1 protein expression at 12 to 24 hours (Figure 5A). In contrast, VEGF-A165 had no effect on luciferase expression during the 16-hour time course (Figure 7A).
Histamine and serotonin, but not VEGF-A165, activate the TSP-1 promoter, but not directly through TR3. (A) HUVEC were transduced with TSP-1 promoter luciferase and internal luciferase constructs and then were stimulated with histamine (10 μM) (left panel), serotonin (10 μM) (right panel), or VEGF-A165 (10 ng/mL) (both panels) as indicated (n = 6; experiments were repeated 3 times). ***P < .001 vs control, Tukey-Kramer multiple comparisons test. (B) Cell extracts from HUVEC that were not transduced or transduced with LacZ (control) or with TR3 cDNAs were subjected to immunoblotting with antibodies against TSP-1 (top panel), VE-cadherin (middle panel), and β-actin for protein equal loading control (bottom panel). Data are representative of 3 independent experiments.
Histamine and serotonin, but not VEGF-A165, activate the TSP-1 promoter, but not directly through TR3. (A) HUVEC were transduced with TSP-1 promoter luciferase and internal luciferase constructs and then were stimulated with histamine (10 μM) (left panel), serotonin (10 μM) (right panel), or VEGF-A165 (10 ng/mL) (both panels) as indicated (n = 6; experiments were repeated 3 times). ***P < .001 vs control, Tukey-Kramer multiple comparisons test. (B) Cell extracts from HUVEC that were not transduced or transduced with LacZ (control) or with TR3 cDNAs were subjected to immunoblotting with antibodies against TSP-1 (top panel), VE-cadherin (middle panel), and β-actin for protein equal loading control (bottom panel). Data are representative of 3 independent experiments.
TR3 is a transcription factor. Therefore, we analyzed the transcriptional regulatory elements in the TSP-1 promoter but did not find TR3 conserved regulatory elements.37 We also tested whether overexpression of TR3 had an effect on TSP-1 expression. Cell extracts from HUVEC that were not transduced, or were transduced with TR3 or with LacZ cDNAs, were subjected to immunoblot analysis with an antibody against TSP-1. TSP-1 expression was not affected by overexpression of TR3 (Figure 7B, top panel). We had shown previously that TR3 down-regulated VE-cadherin expression.25 Therefore, to demonstrate that the TR3 in our assay was functional, we measured expression of VE-cadherin from the same extracts and found that it was down-regulated as expected by overexpression of TR3 (Figure 7B, middle panel). Thus, histamine and serotonin regulate TSP-1 promoter activity, but TR3, if involved, acts on TSP-1 by an indirect mechanism.
Discussion
The data presented here provide definitive evidence that histamine and serotonin are potent angiogenic factors at low micromolar concentrations and that, like VEGF-A165, they exert this activity directly through the orphan nuclear receptor and transcription factor TR3/Nur77. Histamine and serotonin both stimulated HUVEC incorporation of 3H thymidine, migration, and tube formation (Figure 1A-D). Furthermore, these responses were inhibited by different selective antagonists of histamine or serotonin receptors, but not by the VEGFR-2 inhibitor SU1498 (Figure 1, Table 1), and were greatly reduced in HUVEC in which TR3 was knocked down by ∼90% (Figure 3A-D). In a likewise fashion, histamine and serotonin slow-releasing pellets induced angiogenesis when implanted in nude mice, and the responses were inhibited by selective histamine or serotonin receptor antagonists but not by SU1498 (Figure 2). Of note, angiogenesis did not develop when pellets were implanted in Nur77−/− mice (Figure 3E). Although the angiogenic responses induced by histamine and serotonin pellets initially mimicked those induced by VEGF-A, they were transitory and began to decline after ∼10 days. Investigating the mechanisms responsible, we found that histamine and serotonin down-regulated TSP-1 expression for a time in both cultured HUVEC (Figure 5A,C) and in vivo in the tissues surrounding the implanted pellets (Figure 5B-C). The mechanism of TSP-1 downregulation is unknown but could reflect changes in messenger RNA stability. Subsequent restoration of TSP-1 to normal levels, both in cultured HUVEC and in vivo, was attributable to activation of the TSP-1 promoter by both histamine and serotonin (Figure 7A). VEGF-A also down-regulated TSP-1 expression in cultured HUVEC but had limited or no effect on TSP-1 expression at skin sites injected with Ad-VEGF-A164 (Figure 5A-C).
The antiangiogenic recombinant TSP-1–derived protein, 3TSR, strikingly inhibited both histamine- and serotonin-induced HUVEC proliferation, migration, and tube formation (Figure 6A-D). Also, histamine- and serotonin-induced angiogenic responses were strikingly inhibited by local injection of an AAV expressing 3TSR (Figure 6E). Nonetheless, the relationships between histamine, serotonin, TR3/Nur77, and TSP-1 expression remain elusive. Whereas TR3/Nur77 was found to down-regulate VE-cadherin expression and so modulate vascular permeability,25 overexpression of TR3 had no effect on TSP-1 expression (Figure 7B). Additional work will be required to elucidate the pathways by which histamine and serotonin regulate TSP-1 expression.
Although very limited data have implicated serotonin in angiogenesis since the early report of Zauberman,14 many studies have reported a role for histamine in the angiogenesis associated with acute inflammation. The Norrby group15-17 reported that compound 48/80, a mast cell–degranulating agent, induced angiogenesis in peritoneal mesenteries. Mast cells express and, on degranulation release, many bioactive molecules besides histamine, including VEGF-A165,38 but a role for histamine was strongly supported by the finding that compound 48/80–induced angiogenesis was inhibited by H1 and H2 histamine receptor antagonists. These results are similar to ours in which both H1 and H2 antagonists inhibited histamine-induced HUVEC proliferation, migration, and tube formation, as well as histamine pellet–induced angiogenesis (Figure 1 and 2). The kinetics of angiogenesis induced by histamine-releasing pellets are also consistent with earlier reports of angiogenesis induced by compound 48/80 in peritoneal mesenteries. Jakobsson et al39 demonstrated that vascular density and vascular surface area in mesenteries were maximal at 9 days after intraperitoneal injection of compound 48/80 and had regressed by day 16, timing similar to that of the histamine pellet–induced angiogenesis we observed (Figure 4).
Ghosh et al18 also provided evidence that histamine had a role in inflammatory angiogenesis. Using a cotton-string implantation model, they found that angiogenesis was greatly reduced in mice null for histidine decarboxylase, the enzyme responsible for histamine synthesis. They also reported that the H2 blocker cimetidine inhibited angiogenesis in this model; however, in contrast to our work and to that of Norrby et al, a H1 receptor inhibitor was without effect. Also, Ghosh et al18 found that anti-VEGF-A165 antibodies inhibited the angiogenic response in the string assay. Therefore, they attributed the angiogenic effect of histamine to an accumulation of histidine decarboxylase–expressing macrophages, which secreted histamine and so induced VEGF-A165 formation. These data are not in conflict with ours in that histamine- and serotonin-secreting pellets elicited very few macrophages (Figure 4B). Thus, histamine may induce angiogenesis by 2 mechanisms, both of which involve TR3/Nur77 (ie, upregulation of TR3/Nur77 directly or indirectly by first inducing VEGF-A165 expression, which subsequently up-regulates TR3/Nur77.24,25 )
In addition to their long-studied roles in acute inflammation, histamine and serotonin and their receptors have recently been implicated in cancer.8,19,20,40-43 Many reports indicate that both amines are tumor cell mitogens, but, in at least some cases, they have been implicated in tumor angiogenesis. For example, Natori et al43 demonstrated that cimetidine, a histamine H2 receptor inhibitor, inhibited the angiogenic response induced by syngeneic CMT93 colon cancer cells. Also, Nocito et al reported decreased angiogenesis and consequent reduced colon cancer growth in serotonin-deficient mice, which they attributed to an induction of the angiogenesis inhibitor angiostatin.8
In summary, our data add another layer to the complexity of pathologic angiogenesis. Histamine and serotonin, in addition to their many other functions, induce angiogenesis directly by activating the transcription factor TR3/Nur77. After a time, however, histamine and serotonin also inhibit angiogenesis by promoting expression of the angiogenic inhibitor TSP-1. These findings are consistent with the long-held view that histamine and serotonin have roles in acute inflammation and immediate hypersensitivity and suggest that, by truncating angiogenesis, one of their functions may be to prevent these reactions from becoming chronic. On the other hand, to the extent that they also stimulate VEGF-A165 expression in inflammatory cells,18 histamine and serotonin may also have roles in promoting the angiogenesis of chronic inflammation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported in part by research funding from National Institutes of Health grants K01 CA098581 and R01CA133235 (H.Z.); CA-142262, P01 CA-92644, and a contract from the National Foundation for Cancer Research (H.F.D.); and CA-130895 and NS071197 (J.L.).
National Institutes of Health
Authorship
Contributions: L.Q., D.C., E.F.T., S.P., J.L., H.F.D., and H.Z. designed the research. L.Q., D.Z., J.X., X.R., and H.Z. performed the experiments. D.Z., H.F.D., and H.Z. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for J.X. is GlaxoSmithKline R&D China, Shanghai, China.
Correspondence: Huiyan Zeng, PhD, Division of Molecular and Vascular Biology, Department of Medicine, Beth Israel Deaconess Medical Center, and Harvard Medical School, 99 Brookline Ave. RN 270F, Boston, MA 02215; e-mail: hzeng@caregroup.harvard.edu.
References
Author notes
L.Q. and D.Z. contributed equally to this study.