In this issue of Blood, Varettoni et al report on the prevalence of MYD88 L265P mutation in patients with Waldenström macroglobulinemia (WM), IgM-MGUS, and other late B cell lymphoid disorders.1
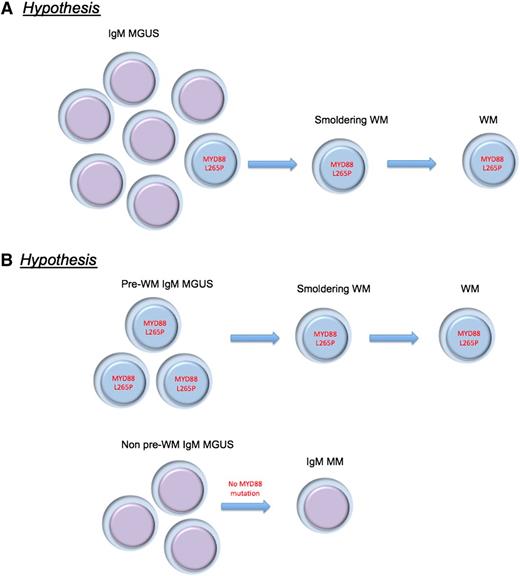
Alternative hypotheses proposed for the MYD88 L265P mutation in IgM-MGUS and WM pathogenesis. (A) MYD88 L265P mutation is a critical and universal progression event from IgM-MGUS to WM. (B) There are various biologic subtypes of IgM MGUS, and the MYD88 L265P mutation is found in the subtype that originates WM.
Alternative hypotheses proposed for the MYD88 L265P mutation in IgM-MGUS and WM pathogenesis. (A) MYD88 L265P mutation is a critical and universal progression event from IgM-MGUS to WM. (B) There are various biologic subtypes of IgM MGUS, and the MYD88 L265P mutation is found in the subtype that originates WM.
The MYD88 L265P mutation, first described in diffuse large B cell lymphoma,2 seems almost universal in WM,3 and it has also been found in other lymphoid disorders such as IgM-MGUS, chronic lymphocytic leukemia, and marginal zone lymphomas, albeit less commonly than in WM.2,4 The authors confirm previous observations made by Treon in identifying MYD88 (L265P), using an unbiased approach, as a disease-defining genetic alteration in WM.3
This study adds to our understanding of disease pathogenesis as it also shows that one-half of patients with IgM MGUS also harbor such mutations, which is an observation made by Landgren and colleagues as well.5 One can envision how detection of the mutation could serve both as a diagnostic adjunct as well as a bona fide therapeutic target. The relative genomic stability of WM6,7 makes targeting the signaling induced by MYD88 (L265P) mutations a top priority, because it is likely that antitumor effects could be seen with development of small molecule inhibitors. Although many therapeutic options exist for patients with WM, the disease remains incurable and a threat to patients’ survival, and better treatments are clearly needed.
Many new questions arise with these observations, however. The mutation is detected, with current methodology, in only 50% of IgM MGUS cases. Two hypotheses seem evident: the mutation is a critical and universal progression event (still an essential component of disease pathogenesis), or there are various biologic subtypes of IgM MGUS as alluded to by the authors (see figure). In contrast to non-IgM MGUS, clonal cells with IgM-producing abilities are probably more likely to reside in other lymphoid tissues outside of the bone marrow, and they could be the niche for IgM production, but apart from testing strategies using bone marrow cells. Careful selection of patients with IgM MGUS cells by flow cytometry or bead selection of cells should be applied to understand whether the genomic makeup of IgM MGUS with or without MYD88 (L265P) mutations is the same entity. This hypothesis is suggested as less likely by the fact that clonal Ig rearrangements were seen in IgM MGUS cases without the MYD88 (L265P) mutation, although this needs to be carefully excluded in future studies.
A more likely explanation is that the mutation is a progression event, leading to a question regarding the founding lesions that drive WM pathogenesis. So far, NGS data from other studies have failed to show other highly prevalent mutations or genetic abnormalities in WM.3 Mutations of NF-κB–related genes have also been reported in WM, but they are not common,6 and deletions of the long arm of chromosome 6 are seen in 50% of cases.8 How can this be reconciled? One possibility is that there are various (perhaps many) pathways by which an aberrant IgM-producing clone can develop in the germinal center, but can remain subclinical (not detectable because of low concentration of IgM monoclonal proteins) or clinically silent (IgM MGUS). But then a MYD88 (L265P) mutation occurs and accelerates (unmasks) such clones leading to the diagnosis of WM, smoldering WM, or even more advanced IgM MGUS. Even if the mutation was found to be subclonal in all IgM MGUS cases, this would still suggest it is a progression event.
Familial predisposition for WM is reported in a smaller subset of cases,9 although it should be mentioned that in some of these families the predisposition includes IgM MGUS. Could the predisposition occur because of normal SNP variants or mutations of MYD88 (L265P)? This is unknown, but it is a question in need of testing. An alternative hypothesis is that the familial predisposition occurs for these pre-WM clones, and the acquisition of MYD88 (L265P) mutations occurs in a stochastic fashion but is common enough that phenotype results in familial clustering. It should also be remarked that WM is associated with a personal and family history of autoimmunity and allergies,10 and, as such, normal variants of other immune regulatory molecules are possible.
In conclusion, these findings once more show the revolutionary power that next-generation sequencing has brought to cancer research. In this golden age of cancer genomic discovery, the MYD88 (L265P) “touch” is happening!
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal