Key Points
In intermediate-risk AML, effect of FLT3 burden is modulated by NPM1 mutation, especially in patients with a low ratio.
Combined evaluation of NPM1 mutation and FLT3-ITD burden might contribute to identify patients who benefit from early allogeneic HSCT.
Abstract
Risk associated to FLT3 internal tandem duplication (FLT3-ITD) in patients with acute myeloid leukemia (AML) may depend on mutational burden and its interaction with other mutations. We analyzed the effect of FLT3-ITD/FLT3 wild-type (FLT3wt) ratio depending on NPM1 mutation (NPM1mut) in 303 patients with intermediate-risk cytogenetics AML treated with intensive chemotherapy. Among NPM1mut patients, FLT3wt and low ratio (<0.5) subgroups showed similar overall survival, relapse risk, and leukemia-free survival, whereas high ratio (≥0.5) patients had a worse outcome. In NPM1wt AML, FLT3-ITD subgroups showed a comparable outcome, with higher risk of relapse and shortened overall survival than FLT3wt patients. Allogeneic stem cell transplantation in CR1 was associated with a reduced relapse risk in all molecular subgroups with the exception of NPM1mut AML with absent or low ratio FLT3-ITD. In conclusion, effect of FLT3 burden is modulated by NPM1 mutation, especially in patients with a low ratio.
Introduction
The presence of FLT3 internal tandem duplication (FLT3-ITD) is associated with an increased risk of relapse (RR) and inferior overall survival (OS) in patients with normal karyotype acute myeloid leukemia (AML), arising as one of the main prognostic factors in this group of patients.1-4 However, the risk conferred by this mutation has been related to specific characteristics, such as the allelic burden, length of the mutation, or specific sequence.4-7 Thus, the proportion of the mutant allele among leukemic population is considered one of the most important features modulating the prognostic impact of the mutation.6,7 Nonetheless, the resulting effect of the FLT3-ITD is not only a consequence of intrinsic characteristics of the mutation but can also depend on the interaction with other mutations, such as WT1 or DNMT3A, which seem to add an adverse effect in patients with FLT3-ITD AML.8-10 Moreover, FLT3-ITD, considered as a secondary mutation involving signaling cell pathways that confer a proliferation advantage to the leukemic clone (type I mutation), is frequently observed in patients harboring NPM1 mutations, associated to a favorable prognosis.11-14 Therefore, the effect of FLT3-ITD burden might depend on the presence or absence of an underlying NPM1 mutation, as recently suggested.15-17 To test this hypothesis we analyzed a large series of patients with a long follow-up from the Spanish cooperative group CETLAM (Grupo cooperativo para el estudio y tratamiento de las leucemias agudas mieloblásticas).
Study design
Patients and treatment
Three hundred three patients up to 60 years old diagnosed with de novo AML of intermediate-risk cytogenetics according to the MRC classification18 and with available material at diagnosis were included. Patients were treated according to CETLAM trials since 1994 (AML-94, n = 15; AML-99, n = 91; and AML-03, n = 197). Details of the treatment that was received are provided in supplemental Table 1 on the Blood website.
Analysis of NPM1, FLT3-ITD, and FLT3-ITD/wt allelic burden
All samples were obtained at diagnosis after written informed consent in accordance with the Declaration of Helsinki. All the experiments were approved by the Ethics Committee of each institution. Detection of NPM1 and FLT3-ITD mutations were performed on genomic DNA as previously described,4,19 with labeled primers and analyzed by fragment analysis (3130XL Genetic Analyzer; Applied Biosystems). FLT3-ITD/wt allelic burden was calculated as the ratio of the area under the curve of mutant and wild-type alleles (FLT3-ITD/FLT3wt). In cases with >1 mutation, all FLT3-ITD were summed.
Statistical methods
Characteristics between groups were compared using the χ2 test and Fisher exact test for categorical variables and the Mann-Whitney U test for continuous variables. The median value of continuous variables was used to dichotomize them for prognostic analyses. OS was calculated from diagnosis to death, whereas leukemia-free survival (LFS) was calculated from complete response (CR) to death or relapse; both functions were estimated with the Kaplan-Meier method. RR was estimated using the cumulative incidence (CI) method, defining relapse as the main event and death without relapse as a competitive event. For calculating RR, patients who received an allogeneic stem cell transplantation (alloHSCT) in CR1 were censored at date of transplantation. Comparisons of OS and LFS between groups were performed with a log-rank test, whereas the Gray statistic was used to calculate RR.20 Multivariate analysis for survival was performed using the Cox proportional hazards model. Variables analyzed in univariate and multivariate analyses for all outcomes included gender and age, hematologic parameters at diagnosis (white blood cell count [WBC], platelet, percentage of bone marrow blasts), and main cytogenetic and molecular features (NPM1 and FLT3-ITD mutational status). Statistical analyses were performed using software packages SPSS version 18.0 and Windows 95/NT (SPSS Inc, Chicago, IL), except RR analysis and univariate and multivariate analyses using time-dependent covariates, which were performed with R version 2.13.1.
Results and discussion
Characteristics of patients
The main characteristics of patients are summarized in supplemental Table 2. FLT3-ITD and NPM1 mutations were determined in 303 patients and were detected in 94 (31%) and 161 (53%) patients, respectively, with a significant association between them (65 patients harbored both mutations, P < .001). Patients with FLT3-ITD, compared with FLT3wt, presented with higher WBC and bone marrow blast infiltration, lower platelet count, and normal karyotype in a higher proportion of cases. The median value of the FLT3-ITD/wt ratio was 0.61 (range: 0.033-7.515) without differences between NPM1wt and NPM1mut groups (0.59 vs 0.652; P = .4). Of note, WBC at diagnosis increased progressively in patients without FLT3-ITD, with a FLT3-ITD/wt ratio between 0 and 0.5 and with a FLT3-ITD/wt ratio >0.5 (11.5 × 109/L vs 39.7 × 109/L, P = .002; and 39.7 × 109/L vs 90 × 109/L, P = .01, respectively).
Outcome and prognostic factors
Overall, CR rate was 85%, and OS and LFS at 5 years were 43 ± 3% and 46 ± 3%, respectively. The only factor predictive of CR achievement was a lower WBC at diagnosis (91% vs 79%; P = .004). Univariate and multivariate prognostic studies are summarized in Table 1 and supplemental Table 3. FLT3-ITD/wt ratio, analyzed as a continuous variable, showed prognostic value for all end points. To further confirm the prognostic value of the FLT3-ITD/wt ratio, we subdivided our cohort into 3 groups: FLT3wt, FLT3-ITD/wt ratio <0.5 (low ratio), and FLT3-ITD/wt ratio ≥0.5 (high ratio). Different thresholds of the FLT3-ITD/wt ratio have been proposed, although heterogeneity in methods of determination and therapy administered among studies makes their comparison difficult and probably reflects a continuous effect of the FLT3-ITD/wt ratio. Our choice of 0.5 as the cutoff value was based on the maximum clinical prognostic impact derived from this threshold. In the overall series, this cutoff level resulted in a strikingly different RR in patients with FLT3-ITD, with a CI of relapse of 49 ± 10% and 82 ± 7% in the low and high ratio groups, respectively (P = .0075). These differences, however, did not translate in different OS, probably because of the effect of alloHSCT.
Summary of prognostic factors for relapse incidence, survival, and leukemia-free survival identified in the multivariate analysis
| Variable . | OS . | RR . | LFS . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Age* | 1.771 | 1.287-2.436 | <.001 | 1.147 | 0.779-1.69 | NS | 1.482 | 1.047-2.089 | .027 |
| WBC† | 1.429 | 1.021-2.0 | .038 | 1.22 | 0.779-1.910 | NS | 1.032 | 0.71-1.499 | NS |
| FLT3 (ITD vs wild type) | 1.854 | 1.315-2.614 | <.001 | 2.395 | 1.569-3.654 | .000051 | 1.76 | 1.169-2.59 | .004 |
| NPM1 (mut vs wild type) | 0.676 | 0.484-0.944 | .022 | 0.542 | 0.352-0.835 | .0054 | 0.739 | 0.512-1.067 | NS |
| NPM1mut/FLT3wt‡ | 0.601 | 0.422-0.858 | .005 | 0.488 | 0.313-0.76 | .0015 | 0.615 | 0.418-0.905 | .014 |
| FLT3-ITD ratio§ | 1.274 | 1.124-1.444 | <.001 | 1.604 | 1.427-1.8 | 2 × 10−15 | 1.363 | 1.162-1.598 | <.001 |
| Variable . | OS . | RR . | LFS . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Age* | 1.771 | 1.287-2.436 | <.001 | 1.147 | 0.779-1.69 | NS | 1.482 | 1.047-2.089 | .027 |
| WBC† | 1.429 | 1.021-2.0 | .038 | 1.22 | 0.779-1.910 | NS | 1.032 | 0.71-1.499 | NS |
| FLT3 (ITD vs wild type) | 1.854 | 1.315-2.614 | <.001 | 2.395 | 1.569-3.654 | .000051 | 1.76 | 1.169-2.59 | .004 |
| NPM1 (mut vs wild type) | 0.676 | 0.484-0.944 | .022 | 0.542 | 0.352-0.835 | .0054 | 0.739 | 0.512-1.067 | NS |
| NPM1mut/FLT3wt‡ | 0.601 | 0.422-0.858 | .005 | 0.488 | 0.313-0.76 | .0015 | 0.615 | 0.418-0.905 | .014 |
| FLT3-ITD ratio§ | 1.274 | 1.124-1.444 | <.001 | 1.604 | 1.427-1.8 | 2 × 10−15 | 1.363 | 1.162-1.598 | <.001 |
All variables analyzed for all reported outcomes (response to induction, OS, RR, and LFS) are described in the text. HR, hazard ratio; NS, not significant; 95% CI, 95% confidence interval.
Below and above the median value: 47 years old.
Below and above the median: 19.9 × 109/L.
NPM1mut/FLT3wt genotype vs all other possibilities.
Considered as a continuous variable.
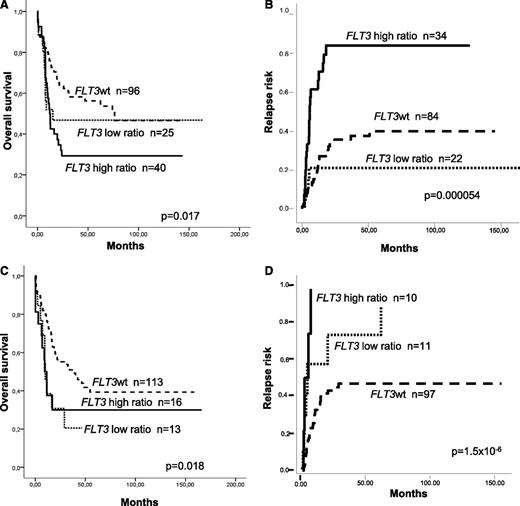
When the analysis was restricted to NPM1mut AML, patients with FLT3wt and low ratio showed similar RR, OS, and LFS (38 ± 6% vs 20 ± 9%, P = not significant; 56 ± 5% vs 47 ± 10%, P = not significant; and 56 ± 6% vs 53 ± 11%, P = not significant, respectively). On the contrary, patients with a high ratio compared with a low ratio and FLT3wt patients showed an increased RR (79 ± 6% vs 20 ± 9% vs 38 ± 9% at 5 years, P = .000054) and shorter OS and LFS (5-year OS: 29 ± 7% vs 47 ± 10% vs 56 ± 5%, P = .017; 5-year LFS: 32 ± 8% vs 53 ± 11 vs 56 ± 6%, P = .025). In contrast, in the NPM1wt cohort, patients with a low ratio showed higher RR and shorter OS compared with those lacking FLT3-ITD (5-year RR: 74 ± 20% vs 48 ± 6%, P = .017; 5-year OS: 20 ± 12% vs 39 ± 6%; P = .014; Figure 1).
Different prognostic value of FLT3-ITD allelic burden according to underlying NPM1 mutational status. Thus, in patients with mutated NPM1, a similar (A) survival and (B) relapse risk were observed between patients without FLT3-ITD and low ratio FLT3-ITD/wt (<0.5), whereas patients with a high FLT3-ITD/wt ratio showed a worse prognosis. In contrast, among wild-type NPM1 AML patients, the presence of FLT3-ITD was associated with a worse outcome compared with those without FLT3-ITD, regardless FLT3-ITD/wt mutational load, both in terms of (C) survival and (D) relapse risk.
Different prognostic value of FLT3-ITD allelic burden according to underlying NPM1 mutational status. Thus, in patients with mutated NPM1, a similar (A) survival and (B) relapse risk were observed between patients without FLT3-ITD and low ratio FLT3-ITD/wt (<0.5), whereas patients with a high FLT3-ITD/wt ratio showed a worse prognosis. In contrast, among wild-type NPM1 AML patients, the presence of FLT3-ITD was associated with a worse outcome compared with those without FLT3-ITD, regardless FLT3-ITD/wt mutational load, both in terms of (C) survival and (D) relapse risk.
We analyzed the potential benefit of alloHSCT in CR1 according to molecular features. First, to avoid a negative bias for patients allocated in the non-alloHSCT arm, we restricted the analysis to patients with a minimum CR duration of 3 months. In the NPM1mut group, high ratio patients experienced a significant reduction of relapse after alloHSCT (5-year RR: 20 ± 13% vs 80 ± 9%, respectively, P = .014), which resulted in a longer OS (22 ± 10% vs 70 ± 14% at 5 years, P = .03). On the contrary, no differences in outcome according to postremission therapy were observed between patients with FLT3wt (5-year OS, RR, and LFS in patients without an allograft and those undergoing alloHSCT in CR1: 64 ± 7% vs 79 ± 11%, P = .296; 35 ± 7% vs 20 ± 11%, P = .49; and 58 ± 7% vs 73 ± 11%, P = .44, respectively) or low ratio FLT3-ITD/wt (5-year OS, RR, and LFS: 67 ± 16% vs 56 ± 17%, P = .873; 22 ± 15% vs 19 ± 20%, P = .566; and 67 ± 16% vs 56 ± 17%, P = .685, respectively). In the NPM1wt subgroup, alloHSCT in CR1 was associated with a significant reduction of RR in patients with FLT3-ITD (5-year CI: 42 ± 14% vs 100%, P = .00016) and showed a similar trend in the FLT3wt subgroup (5-year CI: 25 ± 10% vs 45 ± 7%, P = .08), resulting in a better LFS of the entire NPM1wt cohort (5-year LFS: 35 ± 7% vs 62 ± 8%, P = .032). The effect of alloHSCT in CR1 was also analyzed as a time-dependent variable in molecular risk categories defined following previous results: a low-risk category comprising NPM1mut patients with FLT3wt or a low ratio and a high-risk category comprising patients with NPM1wt and/or high ratio FLT3-ITD. Thus, whereas alloHSCT did not modify the outcome of patients within the low-risk category, high-risk patients had a longer OS (hazard ratio = 0.51, 95% confidence interval: 0.29-0.88, P = .01) and LFS (hazard ratio = 0.48, 95% confidence interval: 0.29-0.79, P = .004; supplemental Figure 1).
Prognostic relevance of FLT3-ITD/wt allelic burden might be based on biological grounds: highest FLT3-ITD/wt ratios correspond to a homozygous state, usually because of a process of uniparental disomy, whereas FLT3-ITD with a low ratio might have arisen in a minor subclone, perhaps occurring at a later stage of the leukemogenic process.21 Thus, the prognosis of these latter patients might not be determined as much by constitutional activation of FLT3 pathway as by other relevant leukemogenic mutations, such as NPM1 mutation. This prognostic interaction between NPM1 mutational status and FLT3-ITD/wt ratio, confirmed in our series, has only been identified in 2 previous studies,15,16 although FLT3-ITD/wt ratio in these 2 studies was determined using cDNA instead of usual determination on genomic DNA.
Despite some controversial results, a general recommendation to perform an early alloHSCT in younger AML patients with FLT3-ITD AML has been established.6,14,22-25 Our finding of a subset of patients with NPM1 mutation and low ratio FLT3-ITD/wt with a low RR, even in patients not submitted to alloHSCT, would prompt to refine this general recommendation and consider their inclusion in a category of AML with favorable genotype. Nonetheless, this observation warrants confirmation in prospective studies, given its potential clinical relevance in the design of postremission therapeutic strategies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all the physicians and data managers from centers of the CETLAM group network for their active contribution to this study.
This work was partially supported by grants N-2004-FS041085, PI080158, RD06/0020/0004, RD06/0020/0101, and EC07/90065 from Instituto de Salud Carlos III (ISCIII), the Spanish Ministry of Health, Spain, and grant 2009-SGR-168 from Pla de Recerca de Catalunya. M.P. holds a grant Río Hortega from ISCIII (CM08/00027).
Authorship
Contribution: This study was designed by M.P. and J.E. Laboratory and data analysis was performed by M.P., J.N., M.C., M.T., D.C., and J.E. Clinical samples and data were provided by S.B., J.N., J.M.R., M.T., M.D.-B., R.D., L.E., R.G., M.P.Q.d.L., O.S., J.B., C.P., J.M.M., M.H., J.S., and J.E. The manuscript was drafted by M.P. and J.E.; all authors contributed to the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jordi Esteve, Hematology Department, Hospital Clínic, Villarroel 170, 08036 Barcelona, Spain; e-mail: jesteve@clinic.ub.es.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal