Key Points
Induction followed by allo-transplantation can achieve long-term disease control in select patients with AML arising from a Ph-MPN.
In this population, transplant should be the goal in patients treated with curative intent, as induction alone provides limited benefit.
Abstract
Leukemic transformation (LT) is a rare but fatal complication of Philadelphia-negative myeloproliferative neoplasms (MPNs) for which optimal treatment strategies are not known. At our center, we have adopted a treatment approach for LT where patients within the transplant age group who have a reasonable fitness level are treated with curative intent and offered induction chemotherapy. Subsequently, those who respond and have a suitable donor are considered for allogeneic hematopoietic cell transplantation (HCT). In this study, we evaluated the clinical outcomes of this treatment approach in 75 patients with LT. The 2-year overall survival (OS) from the time of LT was 15%. A total of 39 patients (52%) were treated with curative intent (induction ± HCT) and had a 2-y OS of 26% compared with 3% in those noncuratively treated (P < .0001). In the curative intent group, 18 individuals (46%) achieved complete remission (CR) or CR with incomplete recovery and 12 (31%) reverted to a chronic MPN phase, with 17 patients undergoing HCT. Survival of patients posttransplant was significantly improved compared with those who responded to induction but were not transplanted (2-y OS of 47% vs 15%; P = .03). Thus, induction chemotherapy followed by HCT has the potential for long-term disease control in select patients with LT preceded by a MPN.
Introduction
The Philadelphia-negative myeloproliferative neoplasms (MPNs) are a group of phenotypically related clonal hematopoietic diseases characterized by the overproduction of mature myeloid blood cells and a prolonged clinical course. Transformation to acute myeloid leukemia (AML) occurs in ∼5% to 10% of cases after 10 y and is associated with exceptionally poor prognosis.1-5 At present, our understanding of the optimal treatment strategy for patients who undergo leukemic transformation (LT; also referred to as MPN-blast phase5 ) is limited. In a retrospective analysis of 91 patients with AML arising from myelofibrosis, the median survival from the time of LT was 2.6 mo. Among patients who were treated with induction chemotherapy, reversion to a chronic myelofibrotic state occurred in 41%. These remissions were short lived, however, with a median survival of 3.9 mo, comparable with those who were treated with supportive care or low-intensity chemotherapy.1 Similar outcomes were found in a retrospective analysis of 74 LT patients, where induction resulted in complete remission (CR) in 46% of patients, but the median survival was only 5 mo.2 At present, the best outcomes in this patient population appear to be restricted to those few individuals who have undergone allogeneic hematopoietic cell transplantation (HCT).2,6-9 However, the available data addressing the efficacy of this approach are limited not only by the small number of reported patients, but also by the heterogeneity of the clinical status of those individuals who underwent transplant.
At our institution, a systematic treatment approach for patients with LT has been established by the Leukemia and Blood and Marrow Transplant programs. All individuals within the transplant age group who have a reasonable fitness level are offered induction therapy. Subsequently, those who achieve CR or revert back to a chronic MPN (cMPN) state are considered eligible for HCT if a suitable related or unrelated donor is available. Alternatively, those who are not candidates for the aforementioned approach are offered supportive therapy including clinical trials. Here, we report the results of a retrospective review of 75 consecutive patients who were treated according to this strategy.
Methods
Patients
This study was approved by the Princess Margaret Hospital Cancer Registry and Data Access Committee and the University Health Network research ethics board (REB# 11-0461-CE). From a database of leukemia patients maintained at the Princess Margaret Cancer Centre, individuals assessed between January 1998 and July 2011 and labeled as having dual diagnoses of an MPN and AML were identified. These basic search parameters yielded 101 results, and the corresponding patient charts were manually reviewed. Patients were confirmed to have polycythemia vera, essential thrombocytosis (ET), primary myelofibrosis, postpolycythemia myelofibrosis, postessential thrombocythemia myelofibrosis, or MPN-unclassifiable (MPN-U) according to the World Health Organization and International Working Group for Myelofibrosis Research and Treatment criteria.10,11 Twenty-six patients who did not meet these diagnostic criteria or who had alternative diagnoses, including myelodysplastic syndrome, chronic myelogenous leukemia, acute panmyelosis with myelofibrosis, chronic neutrophilic leukemia, chronic eosinophilic leukemia, or systemic mastocytosis, were excluded from our study cohort. LT was defined as ≥20% myeloid blasts in either the bone marrow (BM) or peripheral blood (PB). In addition to 73 patients who met the formal definition of LT, 2 patients who developed myeloid sarcoma post-MPN were included in our study population. Cytogenetic data were available for 61 of 75 patients at the time of LT (81%) and were analyzed using the revised Medical Research Council (MRC) prognostic classification system.12 Monosomal karyotypes were defined according to established criteria.13
Treatment
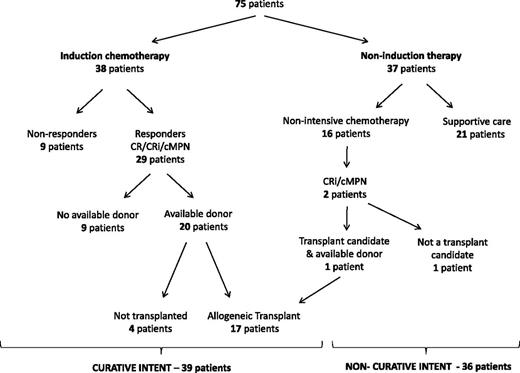
Patients were broadly subdivided into those treated with curative intent (intensive induction chemotherapy ± HCT; n = 39) or noncurative intent (nonintensive chemotherapy or supportive care; n = 36). Those treated as part of clinical trials or with single agent chemotherapy (azacitidine, n = 3; decitabine, n = 3; low dose ara-C, n = 5; and others) were labeled as receiving nonintensive chemotherapy. Patients treated with some combination of standard medical care, transfusional support, and cytoreduction with hydroxyurea (but no other chemotherapeutic agent) were labeled as receiving supportive therapy alone. The reasons for proceeding with treatment with noncurative intent were recorded prospectively in patients diagnosed after 2005; in all others, the reasons were extracted from patient charts.
Induction regimens included: 3+7 (daunorubicin 60 mg/m2 IV bolus daily × 3 d with cytarabine [ara-C] 200 mg/m2 via continuous IV infusion daily × 7 d; n = 25), NOVE-HIDAC (mitoxantrone 10 mg/m2 IV bolus on d 1-5, etoposide 100 mg/m2 IV for 1 h on d 1-5, and cytarabine 1000 mg/m2 IV × 4 doses on d 6 and 7; n = 12), and an acute promyelocytic leukemia induction protocol (all-trans-retinoic acid 45 mg/m2/d for 28 d with amasacrine and cytarabine; n = 1). For patients ≥ 60 y of age who received 3+7, the dose of cytarabine was reduced to 100 mg/m2 via continuous IV infusion. In patients with a left ventricular ejection fraction <50%, amasacrine was used in place of daunorubicin or mitoxantrone.
Responses to induction therapy were defined as one of: CR (BM blasts <5%; absence of MPN features in BM other than fibrosis; absence of PB blasts; absolute neutrophil count ≥1.0 × 109/L; platelet count ≥100 × 109/L; red blood cell and platelet transfusion independence), CR with incomplete recovery (CRi; similar to CR but not meeting either the PB count or transfusion criteria), reversion to cMPN (leukemic blasts <5% in the BM; persistent fibrosis and other MPN features on BM biopsy), resistant disease (BM blasts >5%), or induction-related death (death within 7 d of starting treatment or during therapy-induced BM hypoplasia).
For patients who underwent HCT, the intensity of conditioning was defined by the Center for International Blood and Marrow Transplant Research criteria.14 Hematopoietic recovery post-transplant, acute graft-versus-host disease, and chronic graft-versus-host disease were defined as previously described.15
Statistical analysis
The baseline characteristics of patients were described using the median, range, and 95% confidence interval and were compared by Student’s 2-tailed t test, χ-square analysis, and Fisher’s exact test, as appropriate. Overall survival (OS) was defined as the time between diagnosis of LT and either patient death or the time of last follow-up and was evaluated using the Kaplan-Meier method. For univariate analysis, Mantel-Cox log-rank tests were used to examine the association between baseline characteristics and OS. A landmark survival analysis was performed by comparing OS between individuals who underwent HCT (n = 17) and 12 patients who underwent induction, achieved CR/CRi/cMPN, but were not transplanted and survived at least 184 d, the median time to transplantation. Data analysis was performed using Statistical Analysis Software (SAS) Version 9.2, with statistical significance defined as P ≤ .05.
Results
Patient characteristics
Between January 1998 and July 2011, 75 patients with LT and a preceding diagnosis of a Philadelphia-negative MPN were identified. The baseline characteristics of this population and their flow through our treatment algorithm are outlined in Table 1 and Figure 1, respectively. A total of 39 patients (52%) were treated with curative intent (induction chemotherapy ± HCT) while the remainder were treated with noncurative intent (nonintensive chemotherapy or supportive care). Of note, a single patient, diagnosed with acute megakaryocytic leukemia post-myelofibrosis, took an atypical route to HCT (Figure 1). Following treatment with hydroxyurea alone, this individual unexpectedly reverted to a chronic myelofibrotic state, a finding confirmed by serial BM examinations. Due to this favorable response, the patient proceeded directly to HCT despite not undergoing formal induction therapy and is included as part of the curative intent cohort.
Baseline characteristics of patients within the whole study cohort as well as the subgroups treated with curative and noncurative intent
| . | Whole cohort (n = 75) . | Curative intent (n = 39) . | Noncurative intent (n = 36) . | P value, curative vs noncurative . |
|---|---|---|---|---|
| Male sex, n (%) | 43 (57) | 24 (62) | 19 (53) | NS |
| MPN diagnosis, n (%) | ||||
| PV/PPV-MF | 21 (28) | 10 (26) | 11 (31) | NS |
| ET/PET-MF | 23 (31) | 12 (31) | 11 (31) | NS |
| Primary myelofibrosis | 21 (28) | 12 (31) | 9 (25) | NS |
| MPN-U | 10 (13) | 5 (13) | 5 (14) | NS |
| Age at MPN diagnosis, y, median (range) | 57 (18-81) | 53 (18-80) | 64 (34-81) | .0002 |
| Age at LT, y, median (range) | 65 (36-89) | 57 (36-88) | 72 (54-89) | <.0001 |
| Latency to LT, y, median (range) | 3.9 (0.14-43) | 2.3 (0.14-33) | 5.8 (0.21-43) | NS |
| MPN JAK2 status, n (%) | n = 31 | n = 19 | n = 12 | |
| Positive | 13 (42) | 10 (53) | 3 (25) | NS |
| Negative | 18 (58) | 9 (47) | 9 (75) | |
| MPN therapies, n (%) | ||||
| 2 or less | 51 (68) | 29 (74) | 22 (61) | NS |
| 3 or more | 24 (32) | 10 (26) | 14 (39) | NS |
| Hydroxyurea | 41 (55) | 18 (46) | 23 (64) | NS |
| Anagrelide | 10 (13) | 7 (18) | 3 (8) | NS |
| Chemotherapy | 5 (7) | 0 | 5 (14) | .02 |
| ECOG at LT | n = 74 | n = 38 | n = 36 | |
| 0 or 1 (%) | 56 (76) | 35 (92) | 21 (58) | .0007 |
| ≥2 (%) | 18 (24) | 3 (8) | 15 (42) | |
| Hemoglobin, g/L | n = 73 | n = 37 | n = 36 | |
| Median (range) | 92 (51-177) | 95 (55-177) | 90.5 (51-128) | NS |
| <100, n (%) | 48 (66) | 22 (59) | 26 (72) | NS |
| ≥100, n (%) | 25 (34) | 15 (41) | 10 (28) | |
| White blood cells, ×109/L | ||||
| Median (range) | 18.5 (1-278) | 14.0 (1-213) | 28.8 (1-278) | NS |
| <20, n (%) | 38 (51) | 22 (56) | 16 (44) | NS |
| ≥20, n (%) | 37 (49) | 17 (44) | 20 (56) | |
| Platelets, ×109/L | n = 74 | n = 38 | n = 36 | |
| Median (range) | 69 (1-1800) | 87.5 (6-1800) | 42.5 (1-433) | NS |
| <100, n (%) | 48 (65) | 22 (58) | 26 (72) | NS |
| ≥100, n (%) | 26 (35) | 16 (42) | 10 (28) | |
| Blasts in PB, % | n = 73 | n = 37 | n = 36 | |
| Median (range) | 26 (0-96) | 20 (0-80) | 27.5 (1-96) | NS |
| <20%, n (%) | 25 (34) | 16 (43) | 9 (25) | NS |
| ≥20%, n (%) | 48 (66) | 21 (57) | 27 (75) | |
| Blasts in BM, % | n = 55 | n = 32 | n = 23 | |
| Median (range) | 35 (5-95) | 34.5 (5-80) | 35 (5-95) | NS |
| <50%, n (%) | 32 (58) | 20 (63) | 12 (52) | NS |
| ≥50%, n (%) | 23 (42) | 12 (37) | 11 (48) | |
| Lactate dehydrogenase, U | n = 69 | n = 35 | n = 34 | |
| Median (range) | 616 (129 - 5893) | 566 (144-5893) | 656 (129-2488) | NS |
| Albumin, g/L | n = 67 | n = 35 | n = 32 | |
| Median (range) | 36 (23-47) | 37 (28-47) | 34 (23-45) | .008 |
| Spleen status | n = 66 | n = 33 | n = 33 | |
| Splenectomy | 7 (11) | 3 (9) | 4 (12) | NS |
| Not palpable | 20 (30) | 10 (30) | 10 (30) | NS |
| Splenomegaly | 39 (59) | 20 (60) | 19 (57) | |
| Spleen <10 cm BCM* | 18 (27) | 8 (24) | 10 (30) | NS |
| Spleen ≥11 cm BCM* | 21 (32) | 12 (36) | 9 (27) | |
| Cytogenetics status, n (%) | ||||
| Cytogenetics available | 61 (81) | 32 (82) | 29 (81) | NS |
| Technical limitation | 5 (7) | 2 (3) | 3 (8) | NS |
| Not processed | 9 (12) | 5 (13) | 4 (11) | NS |
| Cytogenetic abnormalities, n (%) | n = 61 | n = 32 | n = 29 | |
| Normal | 22 (36) | 15 (47) | 7 (24) | NS |
| 1 abnormality | 15 (25) | 7 (22) | 8 (29) | NS |
| 2 abnormalities | 7 (11) | 3 (9) | 4 (14) | NS |
| 3 or more abnormalities | 17 (28) | 7 (22) | 10 (34) | NS |
| MRC classification, n (%) | n = 61 | n = 32 | n = 29 | |
| Favorable | 2 (3) | 1 (3) | 1 (3) | NS |
| Intermediate-normal | 22 (36) | 15 (47) | 7 (24) | NS |
| Intermediate-abnormal | 19 (31) | 9 (28) | 10 (34) | NS |
| Adverse | 18 (30) | 7 (22) | 11 (38) | NS |
| Abnormality patterns, n (%) | ||||
| Monosomal karyotype | 12 (20) | 4 (13) | 8 (28) | NS |
| +8 | 10 (16) | 4 (13) | 6 (21) | NS |
| del7q/-7 | 9 (15) | 3 (9) | 6 (21) | NS |
| del5q/-5 | 6 (10) | 3 (9) | 3 (10) | NS |
| del13q/-13 | 3 (5) | 0 | 3 (10) | NS |
| gain/dup 1q | 6 (10) | 3 (9) | 3 (10) | NS |
| gain/dup 9p | 3 (5) | 1 (3) | 2 (7) | NS |
| del17p/-17 | 6 (10) | 2 (6) | 4 (14) | NS |
| . | Whole cohort (n = 75) . | Curative intent (n = 39) . | Noncurative intent (n = 36) . | P value, curative vs noncurative . |
|---|---|---|---|---|
| Male sex, n (%) | 43 (57) | 24 (62) | 19 (53) | NS |
| MPN diagnosis, n (%) | ||||
| PV/PPV-MF | 21 (28) | 10 (26) | 11 (31) | NS |
| ET/PET-MF | 23 (31) | 12 (31) | 11 (31) | NS |
| Primary myelofibrosis | 21 (28) | 12 (31) | 9 (25) | NS |
| MPN-U | 10 (13) | 5 (13) | 5 (14) | NS |
| Age at MPN diagnosis, y, median (range) | 57 (18-81) | 53 (18-80) | 64 (34-81) | .0002 |
| Age at LT, y, median (range) | 65 (36-89) | 57 (36-88) | 72 (54-89) | <.0001 |
| Latency to LT, y, median (range) | 3.9 (0.14-43) | 2.3 (0.14-33) | 5.8 (0.21-43) | NS |
| MPN JAK2 status, n (%) | n = 31 | n = 19 | n = 12 | |
| Positive | 13 (42) | 10 (53) | 3 (25) | NS |
| Negative | 18 (58) | 9 (47) | 9 (75) | |
| MPN therapies, n (%) | ||||
| 2 or less | 51 (68) | 29 (74) | 22 (61) | NS |
| 3 or more | 24 (32) | 10 (26) | 14 (39) | NS |
| Hydroxyurea | 41 (55) | 18 (46) | 23 (64) | NS |
| Anagrelide | 10 (13) | 7 (18) | 3 (8) | NS |
| Chemotherapy | 5 (7) | 0 | 5 (14) | .02 |
| ECOG at LT | n = 74 | n = 38 | n = 36 | |
| 0 or 1 (%) | 56 (76) | 35 (92) | 21 (58) | .0007 |
| ≥2 (%) | 18 (24) | 3 (8) | 15 (42) | |
| Hemoglobin, g/L | n = 73 | n = 37 | n = 36 | |
| Median (range) | 92 (51-177) | 95 (55-177) | 90.5 (51-128) | NS |
| <100, n (%) | 48 (66) | 22 (59) | 26 (72) | NS |
| ≥100, n (%) | 25 (34) | 15 (41) | 10 (28) | |
| White blood cells, ×109/L | ||||
| Median (range) | 18.5 (1-278) | 14.0 (1-213) | 28.8 (1-278) | NS |
| <20, n (%) | 38 (51) | 22 (56) | 16 (44) | NS |
| ≥20, n (%) | 37 (49) | 17 (44) | 20 (56) | |
| Platelets, ×109/L | n = 74 | n = 38 | n = 36 | |
| Median (range) | 69 (1-1800) | 87.5 (6-1800) | 42.5 (1-433) | NS |
| <100, n (%) | 48 (65) | 22 (58) | 26 (72) | NS |
| ≥100, n (%) | 26 (35) | 16 (42) | 10 (28) | |
| Blasts in PB, % | n = 73 | n = 37 | n = 36 | |
| Median (range) | 26 (0-96) | 20 (0-80) | 27.5 (1-96) | NS |
| <20%, n (%) | 25 (34) | 16 (43) | 9 (25) | NS |
| ≥20%, n (%) | 48 (66) | 21 (57) | 27 (75) | |
| Blasts in BM, % | n = 55 | n = 32 | n = 23 | |
| Median (range) | 35 (5-95) | 34.5 (5-80) | 35 (5-95) | NS |
| <50%, n (%) | 32 (58) | 20 (63) | 12 (52) | NS |
| ≥50%, n (%) | 23 (42) | 12 (37) | 11 (48) | |
| Lactate dehydrogenase, U | n = 69 | n = 35 | n = 34 | |
| Median (range) | 616 (129 - 5893) | 566 (144-5893) | 656 (129-2488) | NS |
| Albumin, g/L | n = 67 | n = 35 | n = 32 | |
| Median (range) | 36 (23-47) | 37 (28-47) | 34 (23-45) | .008 |
| Spleen status | n = 66 | n = 33 | n = 33 | |
| Splenectomy | 7 (11) | 3 (9) | 4 (12) | NS |
| Not palpable | 20 (30) | 10 (30) | 10 (30) | NS |
| Splenomegaly | 39 (59) | 20 (60) | 19 (57) | |
| Spleen <10 cm BCM* | 18 (27) | 8 (24) | 10 (30) | NS |
| Spleen ≥11 cm BCM* | 21 (32) | 12 (36) | 9 (27) | |
| Cytogenetics status, n (%) | ||||
| Cytogenetics available | 61 (81) | 32 (82) | 29 (81) | NS |
| Technical limitation | 5 (7) | 2 (3) | 3 (8) | NS |
| Not processed | 9 (12) | 5 (13) | 4 (11) | NS |
| Cytogenetic abnormalities, n (%) | n = 61 | n = 32 | n = 29 | |
| Normal | 22 (36) | 15 (47) | 7 (24) | NS |
| 1 abnormality | 15 (25) | 7 (22) | 8 (29) | NS |
| 2 abnormalities | 7 (11) | 3 (9) | 4 (14) | NS |
| 3 or more abnormalities | 17 (28) | 7 (22) | 10 (34) | NS |
| MRC classification, n (%) | n = 61 | n = 32 | n = 29 | |
| Favorable | 2 (3) | 1 (3) | 1 (3) | NS |
| Intermediate-normal | 22 (36) | 15 (47) | 7 (24) | NS |
| Intermediate-abnormal | 19 (31) | 9 (28) | 10 (34) | NS |
| Adverse | 18 (30) | 7 (22) | 11 (38) | NS |
| Abnormality patterns, n (%) | ||||
| Monosomal karyotype | 12 (20) | 4 (13) | 8 (28) | NS |
| +8 | 10 (16) | 4 (13) | 6 (21) | NS |
| del7q/-7 | 9 (15) | 3 (9) | 6 (21) | NS |
| del5q/-5 | 6 (10) | 3 (9) | 3 (10) | NS |
| del13q/-13 | 3 (5) | 0 | 3 (10) | NS |
| gain/dup 1q | 6 (10) | 3 (9) | 3 (10) | NS |
| gain/dup 9p | 3 (5) | 1 (3) | 2 (7) | NS |
| del17p/-17 | 6 (10) | 2 (6) | 4 (14) | NS |
BCM = palpable below the left costal margin.
NS, not statistically significant, P value > .05; PET-MF, postessential thrombocythemia myelofibrosis; PPV-MF, postpolycythemia myelofibrosis; PV, polycythemia vera.
Flowchart outlining the progression of patients through the treatment algorithm.
Flowchart outlining the progression of patients through the treatment algorithm.
A comparison of the baseline characteristics of patients in the curative and noncurative intent groups is provided in Table 1. As expected, patients treated with curative intent were younger (median age of 57 vs 72 y; P < .0001), had a better performance status (Eastern Cooperative Oncology Group [ECOG] ≤1 in 92% vs 58%; P = .0007), and had a higher serum albumin (37 vs 34 g/L; P = .008) than patients treated with noncurative intent. The reasons for proceeding with a noncurative treatment approach included: age ≥70 y in 26 patients (72%), significant medical comorbidities in 3 patients (8%), poor performance status in 2 patients (6%), personal preference in 4 patients (11%), and the lack of an available donor for HCT in a single patient (3%).
Cytogenetics at LT
A detailed cytogenetic analysis performed at the time of LT was available for 61 of 75 patients (Table 1). Normal cytogenetics were found in 22 of 61 patients (36%), with the remainder having 1 or more karyotypic changes. Of note, 2 individuals had favorable cytogenetics: one patient with inv(16) combined with 2 other alterations and another with a sole t(15;17). Secondary acute promyelocytic leukemia arising in the setting of a prior MPN is a recognized, albeit extremely rare entity.16,17 In an analysis of recurrent cytogenetic abnormalities, additional copies of chromosome 8 emerged as the most common karyotypic change in our patient population (16%). −7/del(7q), −5/del(5q), and −17/del(17p), changes associated with an adverse prognosis in AML, were observed in 9 (15%), 6 (10%), and 6 (10%) individuals, respectively.12 No significant differences in the distribution of cytogenetic abnormalities were noted between the curative and noncurative intent cohorts.
Response to treatment
A total of 38 patients underwent intensive induction chemotherapy, the results of which are summarized in Table 2. After the first course of induction, CR was obtained in 12 patients (32%), CRi was obtained in 2 patients (5%), and 10 (26%) reverted to a cMPN. Of the 9 patients with resistant disease, 6 underwent reinduction, resulting in CR and cMPN in 4 and 1 patients, respectively. Thus, following the induction phase of treatment, 76% of individuals achieved a response, defined as CR (n = 16; 42%), CRi (n = 2; 5%),or cMPN (n = 11; 29%). Significant reductions in BM fibrosis and spleen size were noted in patients who achieved CR/CRi, but complete resolution of these features was rare. cMPN patients all had significant persistent fibrosis as well as other pathologic features suggestive of an underlying MPN in their BM postinduction. A history of myelofibrosis and splenomegaly emerged as the only baseline characteristics predictive of achieving cMPN compared with CR/CRi (P = .02 and 0.03, respectively) (supplemental Table 1).
Outcome of induction chemotherapy
| Regimen . | n . | CR/Cri, n (%) . | cMPN, n (%) . | Resistant disease, n (%) . | Induction-related death, n (%) . |
|---|---|---|---|---|---|
| First induction | |||||
| All | 38 | 14 (37) | 10 (26) | 9 (24) | 5 (13) |
| 3+7 | 25 | 8 (32) | 6 (24) | 9 (36) | 2 (8) |
| NOVE-HIDAC | 12 | 6 (50) | 4 (33) | 0 | 2 (17) |
| Acute promyelocytic leukemia induction | 1 | — | — | — | 1 (100) |
| Second induction | |||||
| NOVE- HIDAC | 6 | 4 (67) | 1 (17) | - | 1 (17) |
| Regimen . | n . | CR/Cri, n (%) . | cMPN, n (%) . | Resistant disease, n (%) . | Induction-related death, n (%) . |
|---|---|---|---|---|---|
| First induction | |||||
| All | 38 | 14 (37) | 10 (26) | 9 (24) | 5 (13) |
| 3+7 | 25 | 8 (32) | 6 (24) | 9 (36) | 2 (8) |
| NOVE-HIDAC | 12 | 6 (50) | 4 (33) | 0 | 2 (17) |
| Acute promyelocytic leukemia induction | 1 | — | — | — | 1 (100) |
| Second induction | |||||
| NOVE- HIDAC | 6 | 4 (67) | 1 (17) | - | 1 (17) |
3+7, daunorubicin and cytarabine; NOVE-HiDAC, mitoxantrone, etoposide and high-dose cytarabine.
The majority of patients who were treated with intensive induction therapy received either 3+7 (n = 25) or NOVE-HIDAC (n=12) as a first-line therapy. Interestingly, a trend toward improved outcomes was noted in individuals treated with NOVE-HIDAC compared with 3+7, with 10 of 12 patients (83%) achieving CR/CRi/cMPN compared with 14 of 25 patients (56%), respectively (P = .10). Moreover, 6 patients who initially failed to respond to 3+7 underwent reinduction with NOVE-HIDAC, with 5 ultimately achieving CR/CRi/cMPN (Table 2). In addition to the induction regimen used, several baseline patient characteristics were predictive of a favorable response to induction (supplemental Table 2). The individuals who achieved CR/CRi/cMPN were younger at the time of LT with a median age of 55.9 y compared with 64.9 y in the nonresponders (P = .003). Moreover, a history of prior splenectomy was associated with a poor response to induction (P = .04); however, no association between spleen size and treatment outcome was identified.
Among the 37 patients who did not undergo intensive induction chemotherapy, 16 received a variety of different nonintensive regimens, often as part of clinical trials. The most commonly used agents were low dose ara-C (n=5), azacitidine (n=3), and decitabine (n=3). The majority of patients treated with nonintensive regimens did not achieve a sustained response to therapy, with 3 notable exceptions. The first, who underwent HCT after achieving CR with hydroxyurea alone, was discussed above. The second patient had FAB-M6 AML post-ET and was treated with combination decitabine and vorinostat. CRi was achieved after 8 cycles of treatment; however, due to advanced age, the patient was not a transplant candidate. Treatment was eventually held after 23 cycles secondary to progressive myelosuppression, and the patient remained leukemia-free until death, 40 mo post-LT. The final patient had MPN-U, which transformed to AML and was treated with azacitidine. This resulted in stable disease with a persistent reduction in PB blast counts until the patient died of infectious complications 15 mo post-LT.
HCT
As per our institution’s treatment strategy, patients who achieved CR/CRi or cMPN following induction therapy were considered for HCT. A donor search was initiated for these individuals as well as for the patient who achieved CR with hydroxyurea alone. A matched sibling or unrelated donor was identified for 21 of 30 patients (70%), with 17 (57%) ultimately undergoing HCT, representing 23% of the whole patient cohort and 44% of patients treated with curative intent. Among the patients with available donors who did not undergo HCT, 3 relapsed prior to transplant and 1 opted not to proceed due to concerns related to morbidity and mortality. A summary of transplant characteristics and outcomes is provided in Table 3. The median time to transplant was 184 d following LT (range, 78-519), and all patients were either in CR or cMPN at the time of transplant. Due to early death, one patient was inevaluable for hematological recovery. All others achieved primary engraftment, with a median time to neutrophil and platelet recovery of 19 and 11 d, respectively.
Characteristics and treatment outcomes of patients treated with HCT
| Number of patients transplanted . | 17 . |
|---|---|
| Donor, n (%) | |
| Matched sibling | 12 (70) |
| Matched unrelated | 4 (24) |
| Mismatched unrelated | 1 (6) |
| Cell type, n (%) | |
| BM | 3 (18) |
| Mobilized PB HSCs | 14 (82) |
| Conditioning, n (%) | |
| Myeloablative | 8 (47) |
| Reduced intensity | 9 (53) |
| Clinical status at transplant, n (%) | |
| CR1 | 8 (47) |
| CR2 | 2 (12) |
| cMPN | 7 (41) |
| Primary engraftment, n (%) | 16 (94) |
| Days to ANC .5 x 109/L (median), n | 19 |
| Days to PLT count 20 x 109/L (median), n | 11 |
| Acute GVHD, n (%) | |
| None | 3 (18) |
| Grade 1-2 | 11 (65) |
| Grade 3-4 | 2 (12) |
| Not applicable | 1 (6) |
| Chronic GVHD, n (%) | |
| None/limited | 4 (24) |
| Extensive | 9 (53) |
| Not applicable | 4 (24) |
| Status at last follow-up, n (%) | |
| Alive | 5 (29) |
| Dead | 12 (71) |
| Cause of death, n (%) | |
| Relapse | 4 (33) |
| Infection | 6 (50) |
| Regimen-related toxicity | 2 (17) |
| Number of patients transplanted . | 17 . |
|---|---|
| Donor, n (%) | |
| Matched sibling | 12 (70) |
| Matched unrelated | 4 (24) |
| Mismatched unrelated | 1 (6) |
| Cell type, n (%) | |
| BM | 3 (18) |
| Mobilized PB HSCs | 14 (82) |
| Conditioning, n (%) | |
| Myeloablative | 8 (47) |
| Reduced intensity | 9 (53) |
| Clinical status at transplant, n (%) | |
| CR1 | 8 (47) |
| CR2 | 2 (12) |
| cMPN | 7 (41) |
| Primary engraftment, n (%) | 16 (94) |
| Days to ANC .5 x 109/L (median), n | 19 |
| Days to PLT count 20 x 109/L (median), n | 11 |
| Acute GVHD, n (%) | |
| None | 3 (18) |
| Grade 1-2 | 11 (65) |
| Grade 3-4 | 2 (12) |
| Not applicable | 1 (6) |
| Chronic GVHD, n (%) | |
| None/limited | 4 (24) |
| Extensive | 9 (53) |
| Not applicable | 4 (24) |
| Status at last follow-up, n (%) | |
| Alive | 5 (29) |
| Dead | 12 (71) |
| Cause of death, n (%) | |
| Relapse | 4 (33) |
| Infection | 6 (50) |
| Regimen-related toxicity | 2 (17) |
GVHD, graft-versus-host disease.
Survival
The median survival following LT was 6.6 mo (range, 0.06-112), with a 2-y OS of 15% (Figure 2A). In univariate analyses, age <60 y at LT, an ECOG performance status of ≤1, a BM blast percentage of <50%, and a serum albumin >32 g/L were all associated with significantly improved survival (Table 4). Individuals with adverse cytogenetics according to the MRC criteria were noted to have decreased survival compared with patients with nonadverse chromosomal changes (median survival of 3.4 vs 9.3 mo; P = .05). Moreover, poorer outcomes were noted in individuals with del7q/-7, del17p/-17, and monosomal karyotypes, all of whom fell within the adverse prognostic group. Patients with 3 or more cytogenetic abnormalities, of which 14 of 17 were classified as having adverse prognosis, were also found to have decreased survival with a median OS of 3.3 mo compared with 9.2 mo in those with 2 or fewer alterations (P = .002).
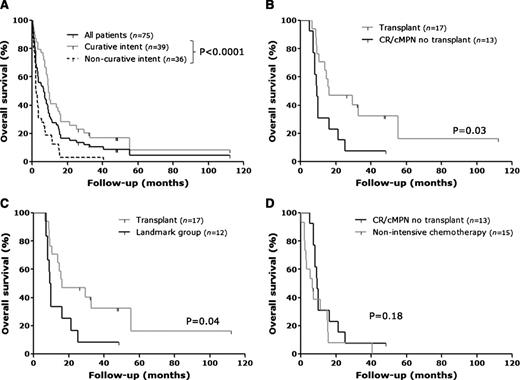
Kaplan-Meier survival analysis for (A) the entire patient cohort (n = 75, black line) and patients treated with curative (n = 39, gray line) and noncurative (n = 36, dashed line) intent. (B) Kaplan-Meier curves comparing OS in patients who underwent allo-SCT (n=17, gray line) and patients treated with induction chemotherapy who achieved either CR/CRi or cMPN (n=13, black line). (C) Landmark Kaplan-Meier analysis comparing patients who underwent allo-SCT (n=17, gray line) and patients who were treated with induction chemotherapy and survived at least 184 d post-LT, the median time to HCT (n=12, black line). (D) Kaplan-Meier analysis comparing patients treated with induction chemotherapy who achieved either CR/CRi or cMPN but were not transplanted (n=13, black line) and patients who were treated with nonintensive chemotherapy (n=15, gray line). For all curves, statistical analysis was performed using the Mantel-Cox log-rank test.
Kaplan-Meier survival analysis for (A) the entire patient cohort (n = 75, black line) and patients treated with curative (n = 39, gray line) and noncurative (n = 36, dashed line) intent. (B) Kaplan-Meier curves comparing OS in patients who underwent allo-SCT (n=17, gray line) and patients treated with induction chemotherapy who achieved either CR/CRi or cMPN (n=13, black line). (C) Landmark Kaplan-Meier analysis comparing patients who underwent allo-SCT (n=17, gray line) and patients who were treated with induction chemotherapy and survived at least 184 d post-LT, the median time to HCT (n=12, black line). (D) Kaplan-Meier analysis comparing patients treated with induction chemotherapy who achieved either CR/CRi or cMPN but were not transplanted (n=13, black line) and patients who were treated with nonintensive chemotherapy (n=15, gray line). For all curves, statistical analysis was performed using the Mantel-Cox log-rank test.
Univariate analysis of determinants of OS duration post-LT
| Parameter . | Whole cohort . | Curative cohort . | ||||||
|---|---|---|---|---|---|---|---|---|
| n . | HR . | 95% CI . | P value . | n . | HR . | 95% CI . | P value . | |
| Baseline characteristics | ||||||||
| Male sex | 43/75 | 0.88 | (1.44-2.67) | NS | 24/39 | 1.20 | (0.60-2.42) | NS |
| Age ≥60 y at LT | 48/75 | 1.95 | (1.20-3.17) | .007 | 15/39 | 1.39 | (0.66-2.93) | NS |
| ECOG ≥2 | 18/74 | 9.54 | (3.93-23.13) | <.0001 | 3/38 | 276 | (14.7-5146) | .0002 |
| Hemoglobin <100 g/L | 48/73 | 1.01 | (0.60-1.67) | NS | 22/37 | 1.05 | (0.51-2.17) | NS |
| WBC ≥20 ×109/L | 37/75 | 0.98 | (0.61-1.6) | NS | 17/39 | 0.90 | (0.45-1.79) | NS |
| Platelets <100 ×109/L | 48/74 | 1.49 | (0.91-2.43) | NS | 22/38 | 1.39 | (0.69-2.79) | NS |
| PB blasts ≥20% | 48/73 | 1.32 | (0.80-2.19) | NS | 21/37 | 1.13 | (0.54-2.33) | NS |
| BM blasts ≥50% | 23/55 | 2.05 | (1.09-3.83) | .03 | 12/32 | 2.65 | (1.07-6.58) | .04 |
| LDH >3× ULN | 33/69 | 0.84 | (0.51-1.40) | NS | 16/35 | 0.55 | (0.26-1.18) | NS |
| Serum albumin <32 g/L | 9/67 | 3.36 | (1.24-9.06) | .02 | 3/35 | 4.49 | (0.73-27.56) | NS |
| Splenectomy | 7/66 | 2.38 | (0.84-6.71) | NS | 3/33 | 6.45 | (0.93-44.78) | NS |
| Splenomegaly | 39/59 | 1.11 | (0.63-1.98) | NS | 20/30 | 0.85 | (0.35-2.06) | NS |
| Cytogenetics | ||||||||
| Normal vs others | 22/61 | 0.81 | (0.47-1.41) | NS | 15/32 | 0.95 | (0.44-2.08) | NS |
| Adverse vs others | 18/61 | 2.03 | (1.01-4.09) | .05 | 7/32 | 1.52 | (0.54-4.31) | NS |
| del7q/-7 | 9/61 | 3.05 | (1.08-8.62) | .04 | 3/32 | 3.35 | (0.60-18.73) | NS |
| del5q/-5 | 6/61 | 2.37 | (0.77-7.29) | NS | 3/32 | 3.35 | (0.60-18.73) | NS |
| del17p/-17 | 6/61 | 4.41 | (1.22-15.91) | .02 | 2/32 | 3.51 | (0.42-29.23) | NS |
| 3 or more abnormalities | 17/61 | 3.29 | (1.56-6.97) | .002 | 7/32 | 3.51 | (1.09-11.27) | .03 |
| Monosomal karyotype | 12/61 | 3.04 | (1.24-7.48) | .02 | 4/32 | 3.80 | (0.81-17.76) | NS |
| Treatment strategy | ||||||||
| Curative approach | 39/75 | 0.31 | (0.18-0.54) | <.0001 | na | na | na | na |
| Transplant | 17/75 | 0.31 | (0.19-0.51) | <.0001 | 17/39 | 0.27 | (0.13-0.56) | .0005 |
| Parameter . | Whole cohort . | Curative cohort . | ||||||
|---|---|---|---|---|---|---|---|---|
| n . | HR . | 95% CI . | P value . | n . | HR . | 95% CI . | P value . | |
| Baseline characteristics | ||||||||
| Male sex | 43/75 | 0.88 | (1.44-2.67) | NS | 24/39 | 1.20 | (0.60-2.42) | NS |
| Age ≥60 y at LT | 48/75 | 1.95 | (1.20-3.17) | .007 | 15/39 | 1.39 | (0.66-2.93) | NS |
| ECOG ≥2 | 18/74 | 9.54 | (3.93-23.13) | <.0001 | 3/38 | 276 | (14.7-5146) | .0002 |
| Hemoglobin <100 g/L | 48/73 | 1.01 | (0.60-1.67) | NS | 22/37 | 1.05 | (0.51-2.17) | NS |
| WBC ≥20 ×109/L | 37/75 | 0.98 | (0.61-1.6) | NS | 17/39 | 0.90 | (0.45-1.79) | NS |
| Platelets <100 ×109/L | 48/74 | 1.49 | (0.91-2.43) | NS | 22/38 | 1.39 | (0.69-2.79) | NS |
| PB blasts ≥20% | 48/73 | 1.32 | (0.80-2.19) | NS | 21/37 | 1.13 | (0.54-2.33) | NS |
| BM blasts ≥50% | 23/55 | 2.05 | (1.09-3.83) | .03 | 12/32 | 2.65 | (1.07-6.58) | .04 |
| LDH >3× ULN | 33/69 | 0.84 | (0.51-1.40) | NS | 16/35 | 0.55 | (0.26-1.18) | NS |
| Serum albumin <32 g/L | 9/67 | 3.36 | (1.24-9.06) | .02 | 3/35 | 4.49 | (0.73-27.56) | NS |
| Splenectomy | 7/66 | 2.38 | (0.84-6.71) | NS | 3/33 | 6.45 | (0.93-44.78) | NS |
| Splenomegaly | 39/59 | 1.11 | (0.63-1.98) | NS | 20/30 | 0.85 | (0.35-2.06) | NS |
| Cytogenetics | ||||||||
| Normal vs others | 22/61 | 0.81 | (0.47-1.41) | NS | 15/32 | 0.95 | (0.44-2.08) | NS |
| Adverse vs others | 18/61 | 2.03 | (1.01-4.09) | .05 | 7/32 | 1.52 | (0.54-4.31) | NS |
| del7q/-7 | 9/61 | 3.05 | (1.08-8.62) | .04 | 3/32 | 3.35 | (0.60-18.73) | NS |
| del5q/-5 | 6/61 | 2.37 | (0.77-7.29) | NS | 3/32 | 3.35 | (0.60-18.73) | NS |
| del17p/-17 | 6/61 | 4.41 | (1.22-15.91) | .02 | 2/32 | 3.51 | (0.42-29.23) | NS |
| 3 or more abnormalities | 17/61 | 3.29 | (1.56-6.97) | .002 | 7/32 | 3.51 | (1.09-11.27) | .03 |
| Monosomal karyotype | 12/61 | 3.04 | (1.24-7.48) | .02 | 4/32 | 3.80 | (0.81-17.76) | NS |
| Treatment strategy | ||||||||
| Curative approach | 39/75 | 0.31 | (0.18-0.54) | <.0001 | na | na | na | na |
| Transplant | 17/75 | 0.31 | (0.19-0.51) | <.0001 | 17/39 | 0.27 | (0.13-0.56) | .0005 |
CI, confidence interval; HR, hazard ratio; na, not applicable; NS, not statistically significant, P value > 0.05; ULN, upper limit of normal.
Within the whole cohort, 39 individuals were treated with curative intent, namely induction chemotherapy ± HCT. These individuals had significantly improved survival compared with those treated with noncurative intent (2-y OS, 25.6% vs 3.1%, median survival of 9.4 vs 2.3 mo; P < .0001; Figure 2A). Within the curative intent group, an ECOG performance status of ≤1 and a BM blast percentage of <50% were found to be associated with improved survival (Table 4). In terms of cytogenetics, only the number of abnormalities held prognostic significance within the curative intent cohort, as individuals with 3 or more chromosomal changes had a median OS of 7.9 mo compared with 13.5 mo in those with 2 or fewer (P = .03).
Among the patients treated with curative intent, 17 underwent HCT. At the time of last follow-up, 5 of 17 were alive and in remission, with a median OS of 47 mo post-LT (range, 26-112). There was no significant difference in survival between individuals who underwent myeloablative vs reduced-intensity conditioning (median OS of 14.6 vs 29.4 mo; P = .77). Death posttransplant was attributable to regimen-related toxicity in 2 patients, infectious complications in 6 patients, and relapsed disease in 4 patients. The median time to relapse was 8 mo (range, 3.9-46.9). In order to assess the impact of transplant upon survival, OS was compared between patients who underwent HCT and those within the curative intent cohort who achieved CR/CRi/cMPN postinduction but were not transplanted. OS was significantly increased in the transplant group, with a 2-y OS of 47% compared with 15% in the nontransplanted group (P = .03; Figure 2B). Baseline characteristics, including age at LT, performance status, and cytogenetics, were comparable between these 2 groups, with the exception of platelet count, which was higher in transplanted group, and BM blast percentage, which was lower (supplemental Table 3). To avoid a time-to-transplant bias, a landmark analysis was performed comparing survival between the transplant cohort and 12 nontransplanted patients within the curative intent group who responded to treatment and survived at least 184 d, the median time to transplant. This showed a significantly increased OS in the transplanted patients compared with the control group (Figure 2C; 2-y OS 47% vs 17%; P = .04). The benefit of transplant as an adjunct to induction therapy was further highlighted when survival was compared between individuals who achieved CR/CRi/MPN postinduction but were not transplanted and those patients who were treated with nonintensive therapy. No significant difference was identified between these 2 groups, with a median survival of 9.4 and 6.6 mo, respectively (Figure 2D; P = .18).
Discussion
Transformation to AML is a rare but fatal complication of MPNs that is associated with short-lived responses to intensive induction therapy and poor survival. Prior studies have suggested that HCT holds some potential for cure, but these were are limited by low patient numbers, significant heterogeneity in remission status at the time of transplant, and the lack of a control arm to ascertain the net benefit of HCT (summarized in Table 5). Our study has established the feasibility of a well-defined treatment approach for these patients, where those who are deemed candidates for HCT are offered intensive induction therapy with the intent of proceeding to transplant if a response is achieved and a suitable donor is identified. Here, we describe the natural history of 75 consecutive patients from our institution who were diagnosed with LT and demonstrate that HCT has the potential for long-term disease control in select individuals. Seventeen patients (23%) underwent transplantation, representing the largest cohort that has been reported to date. In contrast to previous studies, our transplant population included individuals with all of the subtypes of Ph-MPNs (polycythemia vera, ET, primary myelofibrosis, MPN-U, and secondary MF). Moreover, due to our treatment algorithm, the clinical status of patients was relatively homogenous, as 16 of 17 had undergone induction and all were either in remission or in a cMPN state at the time of transplant. In prior reports, the percentages of patients pretreated with induction and in CR/cMPN at the time of transplant have both ranged as low as 20% (Table 5). The importance of achieving leukemic clearance prior to proceeding with transplant, a cornerstone of our treatment approach, has been highlighted in a recent study that showed improved outcomes in LT patients transplanted in CR/cMPN.9
Comparison of the patient characteristics, treatment strategies, and survival outcomes from the key observational studies of LT
| . | . | All patients . | Induction chemotherapy . | HCT . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study* . | Preceeding MPN . | n . | Median OS, mo . | n . | Favorable response, n (%) . | Median OS, mo . | n . | Pretreated with induction, % . | In CR/CRi/cMPN at transplant, % . | 2-y OS, % . |
| 1 | PMF, PPV-MF, PET-MF | 91 | 2.6 | 24 | 10 (42) | 3.9 | 0 | — | — | — |
| 2 | all Ph-MPNs† | 74 | 5 | 41 | 18 (46) | Not reported | 8‡ | 62.5 | 75 | 73 |
| 4 | all Ph-MPNs† | 23 | 4.6 | 20 | 12 (60) | 6 | 3 | 100 | Not reported | Not reported |
| 9 | ET, PV, PMF | 13 | Not reported | 10 | 6 (60) | Not reported | 8 | 100 | 62.5 | 75§ |
| Transplant-focused studies | ||||||||||
| 7 | PMF, PPV-MF, PET-MF | — | — | — | — | — | 14 | 93 | 43 | 49 |
| 8 | PMF, PPV-MF, PET-MF | — | — | — | — | — | 5 | 20 | ≤20 | 53 |
| Present study | all Ph-MPNs† | 75 | 6.5 | 38 | 29 (76) | 9.2 | 17 | 94 | 100 | 47 |
| . | . | All patients . | Induction chemotherapy . | HCT . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study* . | Preceeding MPN . | n . | Median OS, mo . | n . | Favorable response, n (%) . | Median OS, mo . | n . | Pretreated with induction, % . | In CR/CRi/cMPN at transplant, % . | 2-y OS, % . |
| 1 | PMF, PPV-MF, PET-MF | 91 | 2.6 | 24 | 10 (42) | 3.9 | 0 | — | — | — |
| 2 | all Ph-MPNs† | 74 | 5 | 41 | 18 (46) | Not reported | 8‡ | 62.5 | 75 | 73 |
| 4 | all Ph-MPNs† | 23 | 4.6 | 20 | 12 (60) | 6 | 3 | 100 | Not reported | Not reported |
| 9 | ET, PV, PMF | 13 | Not reported | 10 | 6 (60) | Not reported | 8 | 100 | 62.5 | 75§ |
| Transplant-focused studies | ||||||||||
| 7 | PMF, PPV-MF, PET-MF | — | — | — | — | — | 14 | 93 | 43 | 49 |
| 8 | PMF, PPV-MF, PET-MF | — | — | — | — | — | 5 | 20 | ≤20 | 53 |
| Present study | all Ph-MPNs† | 75 | 6.5 | 38 | 29 (76) | 9.2 | 17 | 94 | 100 | 47 |
Reference 6 not included, as no survival analysis was reported.
All Ph-MPNs = PV, ET, PMF, PPV-MF, PET-MF, MPN-U.
Survival data only provided for 8 of 11 patients that underwent HCT; all 8 had either primary or secondary myelofibrosis.
Value is 2-y progression-free survival (OS not provided).
PET-MF, postessential thrombocythemia myelofibrosis; PMF, primary myelofibrosis; PPV-MF, postpolycythemia myelofibrosis.
With this strategy, our transplant cohort had a 2-y OS of 47%, and at the time of last follow-up, 5 of 17 patients remained alive and AML free, with a median survival of 47 mo. These results suggest that induction chemotherapy followed by HCT has curative potential in some individuals with LT. Moreover, our observations, together with those of others, suggest that in this population, induction therapy alone does not meaningfully prolong survival.1,2 As such, at the outset of treatment, transplant should be the goal in all individuals treated with curative intent. Ideally, all LT patients should be referred to centers with expertise in treating this disease, not only to expedite access to transplant programs, but also to facilitate enrollment in clinical trials for those who are not candidates for induction chemotherapy and HCT. Of note, in our study, approximately one-half of LT patients were not deemed fit for a curative intent treatment strategy due to advanced age, comorbid medical conditions, or poor performance status, a proportion that may be underrepresented as referrals for similar patients may not have been initiated from community centers.
Though limited by the number of patients studied and heterogeneity in treatment protocols, hypomethylating agents showed some potential in our patients. In 1 of 3 individuals treated with azacitidine, PB blast counts were stable for 14 mo post-LT, whereas in 1 of 3 patients treated with decitabine, CRi was achieved, resulting in survival for 40 mo. Our findings are in keeping of those from other groups.3,8 Mascarenhas et al8 showed that in patients ineligible for transplant, decitabine can improve survival to 9 mo, while Thepot et al3 reported that azacitidine resulted in a response in 38% of patients with LT, with a median OS of 8 mo. Thus, hypomethylating agents should be considered in individuals ineligible for transplant. Moreover, they require systematic evaluation in curative intent treatment protocols. Potential roles include maintaining disease control while awaiting the outcome of donor searches, acting as adjuvants to standard induction regimens, or serving as substitutes for intensive chemotherapy prior to HCT, a strategy that has recently been shown to have benefit in MDS.18
Another noteworthy observation in this study was a trend toward better responses with NOVE-HiDAC induction chemotherapy compared with the commonly employed 3+7 regimen. Our group has previously reported activity of NOVE-HiDAC in AML patients refractory to 3+7 chemotherapy.19 We have extended this finding to this high-risk population, as responses were achieved in 83% of individuals treated with NOVE-HIDAC as a first-line regimen, compared with 56% with 3+7. Moreover, NOVE-HIDAC resulted in CR/CRi/cMPN in 83% of patients who had previously failed to respond to 3+7. Together, these findings suggest that induction with NOVE-HIDAC may be preferable to 3+7 in patients with LT post-MPN, an observation that will need to be confirmed prospectively in clinical trials.
Aside from treatment strategy, our study also provided novel insights into the baseline patient characteristics that have prognostic value in this population. Age at LT, PB blast percentage, and splenectomy have previously been identified as determinants of survival following LT.2 In keeping with our algorithm, which selected fit patients for curative intent treatment, a lower age at the time of LT, a higher baseline performance status, and an increased serum albumin level, all variables colinear with the curative intent group, were found to be associated with improved survival. Data pertaining to the prognostic value of cytogenetics in this patient population are limited, with alterations of chromosome 17 and the number of cytogenetic abnormalities having been shown to predict survival in separate reports.2,4 In the present study, both were associated with poor prognosis, as was the broader MRC category of adverse cytogenetics. Although prognostic factors identified within the whole cohort of patients provide some value, this group was comprised of individuals treated with regimens ranging from strict supportive care to HCT. By focusing upon those patients treated with curative intent, poor performance status and the presence of 3 or more cytogenetic alterations were found to be predictive of worse OS, though our numbers were low in certain subgroups. A BM blast percentage >50% was also identified, though this association is likely attributable to the transplant cohort, who had a relatively lower level of BM blasts at diagnosis. Interestingly, splenectomy was associated with an unfavorable response to induction (P = .04), and, consistent with this, a trend toward decreased survival (hazard ratio = 6.45, 95% confidence interval = 0.93-44.78; P = .06) in individuals treated curatively. Thus, patients deemed fit to proceed with induction, who present with these features at the time of LT, may benefit from a more intensive treatment approach such as NOVE-HIDAC followed by HCT.
Historically, patients with MPNs who undergo LT have been considered as having a dismal prognosis with limited treatment options. Due to the rarity of this complication, prospective studies, though not impossible, pose a logistical challenge, as even major centers treat few patients with LT each year. The recent development of standardized criteria to assess treatment response in LT will greatly facilitate the execution of multi-center clinical trials.20 However, until this time, retrospective studies will continue to guide clinical practice. This study has built upon previous reports that have suggested that HCT may be beneficial in this patient population. By using a systematic approach to the treatment of these patients, namely induction chemotherapy followed by HCT in responders, we have shown that long-term disease control is achievable in some patients. Thus, every effort should be made to identify patients that are candidates for this treatment approach at the time of diagnosis, with the prompt initiation of donor searches. Those who are not candidates for transplant should be enrolled in clinical trials, as novel hypomethylating agents are emerging as an alternative treatment modality that may hold benefit in this population.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.A.K. and V.G. designed and performed the research, collected, analyzed, and interpreted data, performed statistical analysis, and wrote the manuscript; E.G.A. performed statistical analysis and reviewed the manuscript; K.J.C. reviewed and provided the cytogenetics data; and H.A.M., J.M.B., J.H.L., M.D.M., A.D.S., A.C.S., and K.W.Y. provided the study patients and reviewed the manuscript. All authors approve the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Vikas Gupta, FRCPathLeukemia / Blood and Marrow Transplant Programs, Princess Margaret Cancer Centre, Suite 5-217, 610 University Ave, Toronto, Ontario, Canada, M5G 2M9; e-mail: vikas.gupta@uhn.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal