Key Points
Response to TKIs can be accurately established by measuring the 3-month transcript level.
An additional measurement of the transcript level at 6 months adds very little useful clinical information to the 3-month result.
Abstract
Several groups have shown that that the BCR-ABL1 transcript level measured at 3 or 6 months after starting treatment with tyrosine kinase inhibitors strongly predicts clinical outcomes for patients with chronic myeloid leukemia. In this work, we asked whether the prognostic value of the 3-month transcript level could be improved by combining the 3- and 6-month results. We classified patients treated with imatinib and patients treated with dasatinib according to their transcript levels at 3 months and 6 months. The patients who met the 3-month landmark but failed the 6-month one had outcomes identical to those of patients who met both landmarks, whereas the patients who failed the first landmark but met the second one had prognoses similar to those who failed both landmarks. In summary, early intervention strategies can be based robustly just on the transcript level at 3 months. This trial was registered at www.clinicaltrials.gov as # NCT01460693.
Introduction
Various clinical trials have shown that second-generation (2G) tyrosine kinase inhibitors (TKIs) such as nilotinib and dasatinib induce higher rates of early complete cytogenetic response (CCyR) and deeper molecular responses than imatinib.1,2 However, imatinib has clear advantages over the 2G-TKIs because we have >13 years of experience using this drug and its side-effect profile is well understood.3 Moreover, imatinib is likely to become much cheaper when the patent expires, as it will soon in most countries. For these reasons, there is increasing interest in developing strategies to identify, as early as possible, patients who will not respond optimally to imatinib so that they can be offered an alternative TKI. These “early intervention” strategies may also be applied to patients treated with upfront 2G-TKIs, but then the best alternative therapy is far from clear.
Several groups showed that the BCR-ABL1 transcript level measured at 3 or 6 months after starting TKI therapy strongly predicts achievement of cytogenetic and molecular responses as well as progression-free survival (PFS) and overall survival (OS).4-6 We have also shown that the molecular assessment made at 3 months on imatinib therapy is a better predictor of prognosis than transcript numbers assessed at 6 months or 12 months.4 Here, we investigated whether it is possible to improve the prognostic accuracy of early measurement of transcript levels by combining the 3- and 6-month results.
Study design
For this analysis, which was approved by the UK Central Ethics Committee, we used 3 populations of newly diagnosed chronic myeloid leukemia (CML) in chronic phase patients: 274 seen at our institution treated with imatinib 400 mg daily as described elsewhere,7 a validation sample of 94 patients also treated with imatinib first line at the Royal Liverpool University Hospital,4 and 142 patients treated with dasatinib 100 mg daily as first-line therapy as described elsewhere in the UK SPIRIT 2 study (ClinicalTrials.gov identifier: NCT01460693).5 Patients gave written informed consent for their data to be used in this analysis in accordance with the Declaration of Helsinki. We used standard definitions of CCyR, 4.5-log reduction in the transcript level (MR4.5), and complete molecular response (CMR).5 We used standard statistical methodologies.4,8-11
Results and discussion
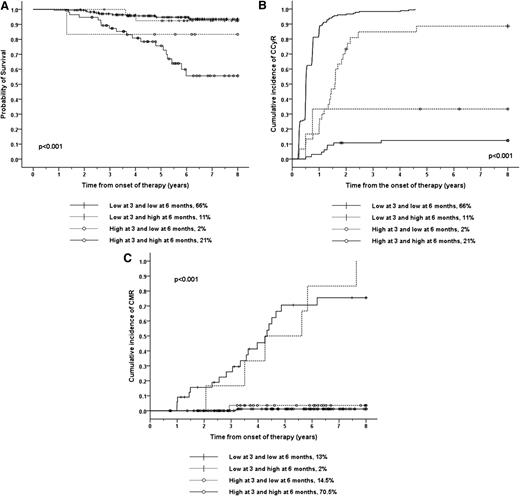
We classified 274 patients treated at Hammersmith Hospital according to their transcript levels. We used as a cut off the previously identified and validated transcript levels that optimally predict for OS at 3 months (lower or higher than 9.8%) and 6 months (lower or higher than 1.67%).4 A total of 66% of the patients had low transcripts both at 3 and 6 months; these patients had an excellent outcome (Figure 1). A total of 21% of the patients had high transcript levels on both occasions; these patients had an outcome significantly worse than those with lower transcripts at both time points. A total of 11% of patients had low transcript levels at 3 months but high transcript levels at 6 months; these patients had a prognosis similar to the patients with low transcripts at both time points although the kinetics of the cytogenetic response in this cohort was slower. Only 2% had high transcript levels at 3 months but low levels at 6 months; these patients had an outcome similar to the patients with high transcript levels at both time points. We found similar results when we used PFS as the outcome criterion (Figure 1). We validate our results using the 94 patients treated at the Royal Liverpool University Hospital (supplemental Table 1).
OS, CI of CCyR, and CI of CMR according to the BCR-ABL1 transcript level at 3 and 6 months. (A) The 8-year probability of OS. The 181 (66%) imatinib-treated patients with low transcript numbers both at 3 months (<9.8%) and at 6 months (<1.67%) had an OS of 93.5% and constitute the reference category for this analysis (group A). The 57 patients (21%) who had high transcript levels on both occasions (group B) had an OS of 55.6% (P < .001). The 30 patients (11%) with low transcript levels at 3 months but high transcript levels at 6 months (group C) had an OS of 92.4% (P = .78). The 6 patients (2%) who had high transcript levels at 3 months but low levels at 6 months (group D) had OS= 83.3% (P = .23). The P value for the comparisons between groups B and C was P = .004 and between groups B and D was P = .39. Similar results were found when we used PFS as outcome criterion; namely, the PFS for patients in the group A was 93.0%. Group B patients had a PFS of 52.5% (P < .001). Group C patients had a PFS of 92.4% (P = .72) and group D patients had a PFS of 83.3% (P = .31). The P value for the comparisons between groups B and C was .001 and between groups B and D was .26. (B) The 8-year CI of CCyR. The 8-year CI of CCyR for patients in groups A, B, C, and D (defined above) was 100%, 14.9% (P < .001), 99.5% (P = .001), and 33.3% (P < .001). The P value for the comparison between groups B and C was <.001 and between groups B and D was .09. (C) The 8-year CI of CMR. We classified the imatinib-treated patients according to whether the transcript level at 3 and 6 months was higher or lower than the previously reported cut off that optimally predicts for this outcome (0.61% for 3 months and 0.21% for 6 months). A total of 34 patients(13%) had low transcripts both at 3 and 6 months, and their 8-year CI of CMR was 75.5%. The CI of CMR for the 6 patients (2%) who had low transcript levels at 3 months but high levels at 6 months was 100% (P = .8). The 193 (70.5%) patients who had high transcript levels on both occasions had a CI of CMR of 1.25% (P < .001) and the 40 (14.5%) patients who had high transcript levels at 3 months but low levels at 6 months had a CI of CMR of 3.6% (P < .001).
OS, CI of CCyR, and CI of CMR according to the BCR-ABL1 transcript level at 3 and 6 months. (A) The 8-year probability of OS. The 181 (66%) imatinib-treated patients with low transcript numbers both at 3 months (<9.8%) and at 6 months (<1.67%) had an OS of 93.5% and constitute the reference category for this analysis (group A). The 57 patients (21%) who had high transcript levels on both occasions (group B) had an OS of 55.6% (P < .001). The 30 patients (11%) with low transcript levels at 3 months but high transcript levels at 6 months (group C) had an OS of 92.4% (P = .78). The 6 patients (2%) who had high transcript levels at 3 months but low levels at 6 months (group D) had OS= 83.3% (P = .23). The P value for the comparisons between groups B and C was P = .004 and between groups B and D was P = .39. Similar results were found when we used PFS as outcome criterion; namely, the PFS for patients in the group A was 93.0%. Group B patients had a PFS of 52.5% (P < .001). Group C patients had a PFS of 92.4% (P = .72) and group D patients had a PFS of 83.3% (P = .31). The P value for the comparisons between groups B and C was .001 and between groups B and D was .26. (B) The 8-year CI of CCyR. The 8-year CI of CCyR for patients in groups A, B, C, and D (defined above) was 100%, 14.9% (P < .001), 99.5% (P = .001), and 33.3% (P < .001). The P value for the comparison between groups B and C was <.001 and between groups B and D was .09. (C) The 8-year CI of CMR. We classified the imatinib-treated patients according to whether the transcript level at 3 and 6 months was higher or lower than the previously reported cut off that optimally predicts for this outcome (0.61% for 3 months and 0.21% for 6 months). A total of 34 patients(13%) had low transcripts both at 3 and 6 months, and their 8-year CI of CMR was 75.5%. The CI of CMR for the 6 patients (2%) who had low transcript levels at 3 months but high levels at 6 months was 100% (P = .8). The 193 (70.5%) patients who had high transcript levels on both occasions had a CI of CMR of 1.25% (P < .001) and the 40 (14.5%) patients who had high transcript levels at 3 months but low levels at 6 months had a CI of CMR of 3.6% (P < .001).
We have previously identified cut offs in the 3- and 6-month transcript levels that predict for the achievement of CMR with maximal sensitivity and specificity, namely 0.61% and 0.21%, respectively4 We therefore wanted to investigate whether it was possible to improve the predictive value of the 3-month assessment by combining the 3- and 6-month results in patients who started treatment with imatinib (Figure 1). We classified the patients using these cutoffs. A total of 13% of patients had low transcripts both at 3 and 6 months; these patients had a very high probability of achieving CMR. The 2% with low transcript levels at 3 months but high levels at 6 months also had a high probability of achieving CMR. On the other hand, both the 70.5% of patients who had high transcript levels on both occasions and the 14.5% of patients who had high transcript levels at 3 months but low levels 6 months had a very low probability of achieving a CMR.
We also investigated whether it was possible to improve the prognostic accuracy of early transcript measurements for patients who started treatment with dasatinib. As previously reported,5 we classified the patients according to transcript level at 3 months (lower or higher than 10%) and 6 months (lower and higher than 1%). As with imatinib, the 6-month transcript level did not improve the predictive power of the 3-month measurement (Table 1). Patients with a low transcript level on both occasions (86.3%) had a very high confidence interval (CI) of CCyR. Patients with a high transcript level at both occasions (7%) fared poorly. As with imatinib, the patients who had low transcripts at 3 months and high transcripts at 6 months (10.9%) also fared well but the kinetics of the response was significantly slower. Only 1 patient had high transcript levels at 3 months and a low level at 6 months.
The 2-year CI of CCyR and MR4.5 in 128 patients treated with dasatinib as first line therapy according to the BCR-ABL1 transcript level at 3 (higher or lower than 10%) and 6 (higher or lower than 1%) months
| Transcript ratio . | n (%) . | CI of CCyR . | CI of MR4.5 . | |
|---|---|---|---|---|
| 3 mo . | 6 mo . | P < .001 . | P < .001 . | |
| <10% | <1% | 104 (81.3) | 100 | 52.9 |
| <10% | >1% | 14 (10.6) | 86.9 | 0 |
| >10% | <1% | 1 (0.8) | — | — |
| >10% | >1% | 9 (7) | 55.0 | 0 |
| Transcript ratio . | n (%) . | CI of CCyR . | CI of MR4.5 . | |
|---|---|---|---|---|
| 3 mo . | 6 mo . | P < .001 . | P < .001 . | |
| <10% | <1% | 104 (81.3) | 100 | 52.9 |
| <10% | >1% | 14 (10.6) | 86.9 | 0 |
| >10% | <1% | 1 (0.8) | — | — |
| >10% | >1% | 9 (7) | 55.0 | 0 |
In this analysis, most of the patients either met both landmarks or failed both landmarks; the former group obtained excellent results while the latter fared poorly. There is a third group of patients who had low transcript levels at 3 months and high levels at 6 months (approximately 10% of those on initial imatinib or dasatinib). These patients had a survival and PFS identical to the patients who had low transcripts at both time points. Their probability of achieving CCyR was also comparable although the kinetics were slower; their median time to obtain CCyR was 18 months for the imatinib cohort (vs 6 months for patients who met both landmarks, P < .001) and 9 months for the dasatinib cohort (vs 3 months, P = .001). Moreover these patients with low levels at 3 months and higher levels are 6 months had a lower probability of obtaining deep molecular responses, although the practical importance of this difference is unclear because it does not seem to affect the probabilities of OS, PFS, or CCyR. The patients who failed the 3-month landmark do not seem to be “rescued” by the fact that they achieved the 6-month one, although the numbers in this group are too small to draw any firm conclusions (2% on imatinib and <1% on dasatinib) and clinical decisions should only be made with caution.
The superior prognostic value of the 3-month over the 6-month measurement may have a biological explanation because TKI therapy spares stem cells12-15 ; the early decline in the transcript level may reflect the decline of mature precursors, which accounts for the response. In our opinion, a more likely explanation is that as the treatment continues other factors, such as adherence to therapy and the need to adjust treatment of toxicity, begin to influence the response and interfere with the prognostic power of the 6-month transcript measurement. We conclude that the prognosis for patients starting TKI can be established accurately by assessing the transcript level only at 3 months and so clinical decisions at this time point can be made with confidence.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients, doctors, nurses, and trial coordinator who make possible the SPIRIT-II trial, in particular Corinne Hedgley.
This work was supported by the National Institute for Health Research, Biomedical Research Centre Funding Scheme, United Kingdom.
Authorship
Contribution: P.N., C.L., M.B., D. Milojkovic, L.W., C.P., S.O., K.R., R.C., J.G., and D. Marin provided patient care and commented on the manuscript. G.G., P.M., and A.R. commented on the manuscript and performed the molecular and cytogenetic studies. D. Marin performed statistical analysis and wrote the manuscript.
Conflict-of-interest disclosure: D. Marin, D. Milojkovic, S.O., and J.G. received research support from Novartis and Bristol Myers-Squibb. The remaining authors declare no competing financial interests.
Correspondence: David Marin, Department of Haematology, Imperial College London, Du Cane Road, London W12 0NN, United Kingdom; e-mail: d.marin@imperial.ac.uk.