To the editor:
The hematopoietic system undergoes rapid changes during embryogenesis; therefore, studying this process requires accurate embryo staging. In the mouse, timed pregnancies can routinely be set and controlled; however, accurate staging of human pregnancies is more problematic, compounded by different ways that gestational age is assessed.
Although developmental biologists calculate gestational age based on the woman’s last ovulation (postovulatory gestational age), clinicians evaluate it from the first day of the last menstrual period (postmenstrual gestational age), using ultrasound measurement of crown-rump length.1 Recording the postmenstrual gestational age of the human embryo largely meets the needs of the medical care. However, it will exceed postovulatory gestational age, which is the true developmental age of the embryo, by 2 weeks in the normal case of a regular menstrual cycle with mid-cycle ovulation and may differ by an unknown duration when the date of ovulation is unknown, which is almost always the case. These ambiguities in gestational age determination introduce significant discrepancies in research into early human embryogenesis.2
The Carnegie staging system, originally described by Streeter3-7 and refined by O’Rahilly and Müller,8 covers the whole human embryonic period (the first 56 days of development) and divides it into 23 Carnegie stages (CSs). Each CS is based on both external and internal morphological criteria and is not directly dependent on gestational age. Human embryos determined clinically to be of the same gestational age can belong to different CSs, and conversely, human embryos belonging to the same CS can be of different gestational ages. Therefore, embryos categorized only according to their gestational ages are not accurately staged with regard to their developmental status.8 The Carnegie system is internationally accepted and provides a staging standard for research purposes. However, it is not uncommon in the literature to see staging such as “22-day-old human embryo” or “6-week-old human embryo,” sometimes without specification of whether this is the postovulatory or postmenstrual gestational age.9-13 On some occasions, damage to the embryo leaves little chance to perform staging using morphological criteria.
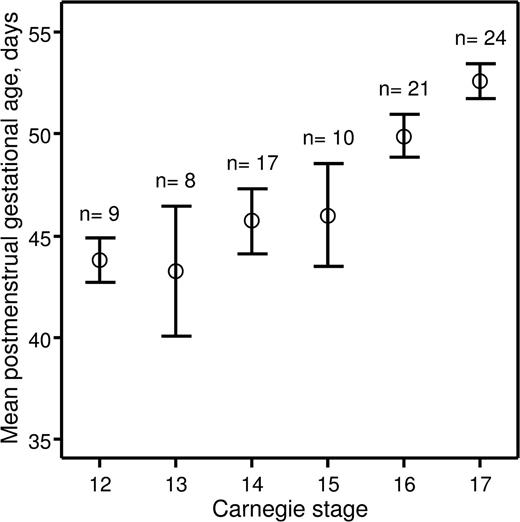
Recently, we investigated hematopoietic stem cell development in the early human embryo.14 The study was approved by the Lothian Research Ethics Committee. Informed consent was provided according to the Declaration of Helsinki. Embryonic specimens were obtained after elective medical termination of pregnancy with written consent and ethical approval of the protocol. Developmental stages were determined according to the Carnegie staging system,8 and we assessed how these correlated with postmenstrual gestational dating. The postmenstrual gestational age was determined for 89 embryos based on ultrasound measurement of crown-rump length. The embryos were split into 6 groups based on developmental stage (CS 12–17). A mean postmenstrual gestational age and a 95% confidence interval were calculated for each of the groups (Figure 1). For embryos categorized as CS 12–15, the 95% confidence intervals of the postmenstrual gestational age overlapped between the 4 groups, indicating that postmenstrual gestational age cannot be used to distinguish between CS 12–15 human embryos. Also, there was no relationship between postmenstrual gestational age of individual CS 12–15 embryos and the CSs to which they were assigned (correlation coefficient r = .251; P = .101). However, CS 16 and 17 embryos could be distinguished based on their postmenstrual gestational age at a 95% confidence level, and there was a direct relationship between postmenstrual gestational age and the CSs to which they belonged (r = .536; P < .001).
Relationship between postmenstrual gestational age and CSs. Human embryos obtained for the present study were split into groups depending on CSs to which they belonged. For each group, a mean postmenstrual gestational age in days (○) and a 95% confidence interval (error bars) were calculated and plotted against corresponding CSs. The number of human embryos obtained for each CS is indicated above the error bars.
Relationship between postmenstrual gestational age and CSs. Human embryos obtained for the present study were split into groups depending on CSs to which they belonged. For each group, a mean postmenstrual gestational age in days (○) and a 95% confidence interval (error bars) were calculated and plotted against corresponding CSs. The number of human embryos obtained for each CS is indicated above the error bars.
These data demonstrate that postmenstrual gestational age cannot be used to accurately stage human embryos under CS 16. This is of particular importance for those working in the field of human developmental hematopoiesis because the first human hematopoietic stem cells emerge at CS 14.14
Authorship
Acknowledgments: The authors thank the patients for donating tissues. The authors also thank research nurses Anne Saunderson, Joan Creiger, and Isobel Morton for patient recruitment. This work was supported by grants from the Leukaemia and Lymphoma Research and the Medical Research Council.
Contribution: A.I. performed the experiments, interpreted experimental data, and wrote the manuscript; S.R., R.A.A., and M.L.T. interpreted experimental data and edited the manuscript; and A.M. directed the study, interpreted experimental data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andrejs Ivanovs, Medical Research Council Centre for Regenerative Medicine, University of Edinburgh, 5 Little France Dr, Edinburgh EH16 4UU, United Kingdom; e-mail: andrejs.ivanovs@ed.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal