Key Points
AhR ligands result in calcium- and ROS-dependent enhancement of mast cell activation.
AhR is critical in controlling mast cell homeostasis.
Abstract
We propose that the aryl hydrocarbon receptor (AhR), a unique chemical sensor, is critical in controlling mast cell differentiation, growth, and function in vitro and in vivo. In antigen-stimulated mast cells, exposure to AhR ligands resulted in a calcium- and reactive oxygen species (ROS)-dependent increase of reversible oxidation in and reduced activity of SHP-2 phosphatase, leading to enhanced mast cell signaling, degranulation, and mediator and cytokine release, as well as the in vivo anaphylactic response. Surprisingly, significant mast cell deficiency was noted in AhR-null mice due to defective calcium signaling and mitochondrial function, concomitant with reduced expression of c-kit and cytosolic STAT proteins, as well as enhanced intracellular ROS and apoptosis. Consequently, AhR-null mast cells responded poorly to stimulation, demonstrating a critical role of AhR signaling in maintaining mast cell homeostasis.
Introduction
The aryl hydrocarbon receptor (AhR) is a ligand-activated transcription factor originally discovered as a receptor for 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD).1 AhR has also been recognized as a receptor for many of the common environmental contaminants and various endogenous ligands, including the tryptophan photoproduct FICZ (6-formylindolo[3,2-b]carbazole), and as a mediator of chemical toxicity.1,2
A considerable amount of evidence suggests that AhR signaling plays a role in the modulation of host immunity. Of note, the recent discovery of the influence of AhR on the balance of regulatory T cells and Th17 cells and its impact on dendritic cell function highlights the potential importance of the AhR-ligand axis in immune regulation.2-10 Further, it has been suggested that AhR signaling is also involved in multiple aspects of normal physiology independent of xenobiotic metabolism, such as modulating host immunity and maintaining cellular homeostasis.6-8,10,11
Mast cells are known to be critical in the regulation of mucosal immunity and allergic and anaphylactic responses. Mast cells can be activated by a multitude of stimuli, resulting in the release of inflammatory mediators and cytokines, typically after the crosslinkage of the high-affinity immunoglobulin E (IgE) receptor FcεRI by surface-bound IgE and antigen, contributing to various pathophysiological events in acute and chronic inflammation.12,13 Recently, 2 observational studies14,15 have suggested a potential role of AhR ligands in regulating mast cell response, although the mechanism remains unclear, and whether AhR plays any endogenous role in controlling mast cell development and function is, at present, unknown. Considering the critical role of mast cells in allergic diseases and their strategic location at the site of tissue mucosa where coexposure of antigens and environmental chemicals often occurs, we therefore hypothesized that there may exist a potential crosstalk between AhR and the high-affinity IgE receptor in controlling the mast cell response and that AhR may be critical in regulating mast cell differentiation and homeostasis.
Methods
All methods and experimental procedures used in this study are described in detail in “supplemental Methods,” including mice, generation of mouse bone marrow–derived cultured mast cells (BMMCs) and human peripheral blood–derived cultured mast cells (HCMCs), assays for mast cell degranulation as well as LTC4 and cytokine measurement, in vivo passive cutaneous anaphylaxis, intracellular calcium measurement, intracellular reactive oxygen species (ROS) production, immunoblotting and immunoprecipitation, analyses of total protein tyrosine phosphatase (PTP) oxidation, oxPTP-antibody–based analyses of PTP oxidation, phosphatase assays, analysis of ubiquitinated proteins, real-time reverse-transcription polymerase chain reaction analysis, mast cell proliferation and differentiation assays, caspase-3 and caspase-7 assays, apoptosis assay, cell-cycle analysis, immunohistochemical staining and quantification of mast cells, analysis of mitochondrial membrane potential and biogenesis, transmission electron microscopy, microarray analysis (GEO accession number GSE44715), and statistical analysis. All experiments were approved by the Animal Care and Use Committee at Johns Hopkins University School of Medicine.
Results
AhR ligands potentiate IgE-mediated mast cell activation
To explore the potential of AhR in regulating mast cell function, mouse BMMCs (c-kit+FcεRI+CD49b−; supplemental Figure 1A), which constitutively expressed AhR (supplemental Figure 1B) and responded to its ligands, were used as a model. Significant, but transient, induction of 2 known AhR targets, cyp1a1 (supplemental Figure 1C) and cyp1b1 (supplemental Figure 1D), members of the phase I enzyme cytochrome P450 family, was observed in mast cells treated with FICZ, a putative endogenous ligand. This effect was AhR specific, since no such effect was found in BMMCs from AhR-null mice (AKO; supplemental Figure 1C-D).
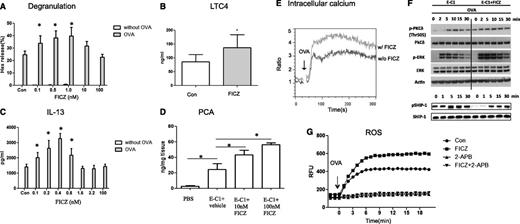
To examine the effects of AhR ligands on FcεRI-mediated mast cell degranulation, BMMCs were sensitized with 1 μg/mL ovalbumin (OVA)-specific IgE monoclonal antibodies (mAbs) plus or minus AhR ligands for varying time points, followed by crosslinkage of surface-bound IgE with antigen (OVA) for 30 minutes. The results showed that while FICZ alone was unable to induce release of β-hexosaminidase (Hex), significantly enhanced levels of IgE/antigen-induced degranulation were found in mast cells treated with low doses of FICZ (Figure 1A) during sensitization, but not at the time of OVA challenge (supplemental Figure 2A); also, FICZ’s effect was not found in AKO mast cells (data not shown). It is noted in our study, however, that higher doses of AhR ligands did not show enhancing effects (inverted U-shaped dose response). This dose response of AhR ligands is consistent with previous reports in other experimental systems.16,17
AhR ligands potentiate IgE-mediated mast cell activation. (A) A low dose of AhR ligands enhances IgE-mediated mast cell degranulation. BMMCs from C57BL/6 mice were sensitized with 1 μg/mL anti-OVA IgE (E-C1) ± FICZ or an equal amount of vehicle as a control (Con) for 16 hours and then stimulated with 10 μg/mL OVA for 30 minutes. Degranulation was monitored by the release of Hex. *P < .05. Data are representative of 3 independent experiments. (B) BMMCs were sensitized with E-C1 ± 1 nM FICZ or an equal amount of vehicle as control (Con) for 16 hours, then washed and stimulated with 10 μg/mL OVA for 30 minutes. *P < .05. Data are representative of 5 independent experiments. (C) AhR ligands enhance IgE-mediated IL-13 production. BMMCs were sensitized with E-C1 ± FICZ or equal amount of vehicle as control (Con) for 16 hours, then washed and stimulated with 10 μg/mL OVA for 6 hours. *P < .05. Data are representative of 3 independent experiments. (D) Analysis of PCA. Mice were injected intradermally with phosphate-buffered saline or 200 ng E-C1 IgE mAbs ± 10 nM or 100 nM FICZ. After 24 hours, 1 mg OVA was administered intravenously together with Evans blue dye, followed by measurement of the extravasation of Evans blue into the skin. In each group, n = 6. *P < .05. Data are representative of 3 independent experiments. (E) Intracellular Ca2+. BMMCs were sensitized with E-C1 ± 1 nM FICZ or equal amount of vehicle as control for 16 hours, loaded with the Ca2+ indicator Fluo-3-AM and Fura red-AM, and resuspended in 2 mM Ca2+ buffer stimulated with 10 μg/mL OVA for 5 minutes. Data are representative of 3 independent experiments. (F) Immunoblotting analysis of whole-cell lysates. BMMCs were sensitized as above, and then the cells were stimulated with 10 μg/mL OVA for the indicated time periods. For detection of phosphorylated and total proteins, 2 equal samples (1 for each) were loaded on the same Criterion XT Bis-Tris Gel (26 wells; Bio-Rad, catalog number 345-0125), so that all the samples were done on 1 gel and transferred to 1 membrane simultaneously. After the transfer to the same membrane, the membrane was cut and each group of samples was detected for phosphorylated and total proteins in parallel. Data are representative of 3 independent experiments. (G) Increased ROS levels in FICZ-exposed, IgE-activated mast cells. BMMCs were sensitized and treated as above, loaded with 5 μM CM-H2DCFDA, and stimulated with 10 μg/mL OVA. The fluorescence was monitored at 30-second intervals using a microplate fluorometer. An inhibitor of Ca2+ signaling (2-APB) was added 30 minutes before OVA stimulation. Data are representative of 3 independent experiments.
AhR ligands potentiate IgE-mediated mast cell activation. (A) A low dose of AhR ligands enhances IgE-mediated mast cell degranulation. BMMCs from C57BL/6 mice were sensitized with 1 μg/mL anti-OVA IgE (E-C1) ± FICZ or an equal amount of vehicle as a control (Con) for 16 hours and then stimulated with 10 μg/mL OVA for 30 minutes. Degranulation was monitored by the release of Hex. *P < .05. Data are representative of 3 independent experiments. (B) BMMCs were sensitized with E-C1 ± 1 nM FICZ or an equal amount of vehicle as control (Con) for 16 hours, then washed and stimulated with 10 μg/mL OVA for 30 minutes. *P < .05. Data are representative of 5 independent experiments. (C) AhR ligands enhance IgE-mediated IL-13 production. BMMCs were sensitized with E-C1 ± FICZ or equal amount of vehicle as control (Con) for 16 hours, then washed and stimulated with 10 μg/mL OVA for 6 hours. *P < .05. Data are representative of 3 independent experiments. (D) Analysis of PCA. Mice were injected intradermally with phosphate-buffered saline or 200 ng E-C1 IgE mAbs ± 10 nM or 100 nM FICZ. After 24 hours, 1 mg OVA was administered intravenously together with Evans blue dye, followed by measurement of the extravasation of Evans blue into the skin. In each group, n = 6. *P < .05. Data are representative of 3 independent experiments. (E) Intracellular Ca2+. BMMCs were sensitized with E-C1 ± 1 nM FICZ or equal amount of vehicle as control for 16 hours, loaded with the Ca2+ indicator Fluo-3-AM and Fura red-AM, and resuspended in 2 mM Ca2+ buffer stimulated with 10 μg/mL OVA for 5 minutes. Data are representative of 3 independent experiments. (F) Immunoblotting analysis of whole-cell lysates. BMMCs were sensitized as above, and then the cells were stimulated with 10 μg/mL OVA for the indicated time periods. For detection of phosphorylated and total proteins, 2 equal samples (1 for each) were loaded on the same Criterion XT Bis-Tris Gel (26 wells; Bio-Rad, catalog number 345-0125), so that all the samples were done on 1 gel and transferred to 1 membrane simultaneously. After the transfer to the same membrane, the membrane was cut and each group of samples was detected for phosphorylated and total proteins in parallel. Data are representative of 3 independent experiments. (G) Increased ROS levels in FICZ-exposed, IgE-activated mast cells. BMMCs were sensitized and treated as above, loaded with 5 μM CM-H2DCFDA, and stimulated with 10 μg/mL OVA. The fluorescence was monitored at 30-second intervals using a microplate fluorometer. An inhibitor of Ca2+ signaling (2-APB) was added 30 minutes before OVA stimulation. Data are representative of 3 independent experiments.
Moreover, increased LTC4 secretion (Figure 1B) and interleukin 13 (IL-13) expression (Figure 1C) were also seen in stimulated mast cells. Similar results were obtained with various AhR ligands, including TCDD and 2-(1H-Indol-3-ylcarbonyl)-4-thiazolecarboxylic acid methyl ester (ITE) (supplemental Figure 2B-C). Also, similar effects of AhR ligands were observed in mast cells stimulated by crosslinkage of dinitrophenyl-specific IgE (SPE-7) with antigen (dinitrophenyl human serum albumin; supplemental Figure 2D). Mast cells remained viable in all conditions (≥95%, data not shown). Moreover, to investigate the possibility of similar enhancing effects on human mast cells, HCMCs were used as a model. The results showed that, consistent with mouse BMMC results, similar enhancing effects of FICZ on mast cell degranulation and cytokine response were noted in HCMCs (supplemental Figure 3) and that the increased levels of IL-13 were abrogated when an AhR antagonist, CH122319 (10 μM), was added to the culture medium (supplemental Figure 3C).
To evaluate whether FICZ had a similar effect in vivo, a model of IgE-mediated passive cutaneous anaphylaxis (PCA) was examined, wherein mice were passively sensitized with phosphate-buffered saline or with OVA-specific IgE Abs plus or minus varying doses of FICZ. An immediate-type allergic reaction was induced 1 day later by intravenous injection of the antigen, OVA, and Evans blue dye. The extravasation of Evans blue dye, indicative of vascular leakage resulting from mast cell activation and anaphylactic response, was analyzed 30 minutes after the induction of PCA. As seen in Figure 1D, a significant enhancement of anaphylactic response was noted in mice treated with either 10 nM or 100 nM FICZ (P < .05), demonstrating the significant functional impact of the FICZ-AhR axis on antigen-induced anaphylaxis in vivo.
Signaling events associated with upregulated mast cell function
The aggregation of FcεRI on the surface of mast cells initiates a complex cascade of signaling events, with elevation of the intracellular calcium concentration ([Ca2+]i) as one of the critical common events. As shown in Figure 1E, while, as expected, an immediate as well as sustained [Ca2+]i increase was observed in mast cells following OVA challenge, a substantial [Ca2+]i enhancement of both phases was noted in FICZ-treated cells. Concomitant with the increased levels of [Ca2+]i, activation of several upstream and downstream signals was noted in FICZ-treated cells upon stimulation with IgE and OVA antigen. Notably, within 2 minutes of stimulation, FICZ-treated cells showed an enhanced level of extracellular signal-regulated kinase (ERK) (Figure 1F), but not c-Jun N-terminal kinase and p38 (data not shown). Further, an increased level of phosphorylated PKCδ was also found, prior to and after OVA challenge, while in FICZ-treated cells there appeared to be a delay in the activation of a negative regulator, SHIP-1 phosphatase (Figure 1F), in mast cell signaling. The lack of the phosphorylated form of SHIP-1 was particularly noted in FICZ-treated mast cells prior to stimulation of the cells with OVA antigen.
Increased ROS levels in FICZ-exposed, IgE-activated mast cells
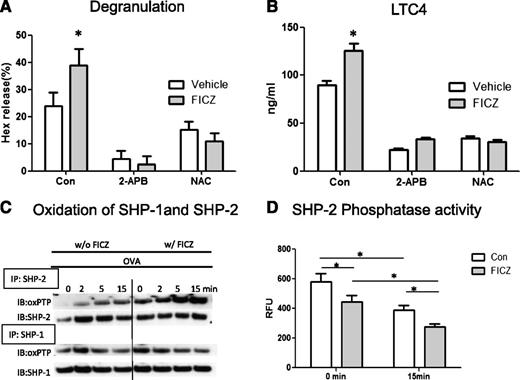
To examine whether AhR ligands regulate the generation of ROS and their subsequent effects on cell activation, intracellular ROS levels were detected with fluorometry. As shown in Figure 1G, while antigen activated mast cells had significantly elevated ROS levels, FICZ-treated cells showed more potent increases in the levels of intracellular ROS following OVA challenge. In fact, at baseline prior to the OVA challenge, treatment of the cells with FICZ alone could potentiate the generation of intracellular ROS (supplemental Figure 4A). Further, as seen in Figure 1G, in the presence of the calcium antagonist 2-aminoethoxydiphenyl borate (2-APB), the increased levels of intracellular ROS were inhibited regardless of FICZ treatment, suggesting that the generation of intracellular ROS was dependent on increased intracellular calcium. Functionally, the generation of [Ca2+]i and ROS contributed to FICZ’s effect on mast cell degranulation and secretion of LTC4. As shown in Figure 2A, the enhanced levels of degranulation in FICZ-treated mast cells were blocked by addition of 2-APB and 2 antioxidants, N-acetyl cysteine and ebselen (supplemental Figure 4B), while, as expected, mast cell response was also inhibited when the cells were stimulated with IgE/antigen alone. Similarly, FICZ’s enhancing effect on mast cell LTC4 release was also inhibited by all 3 inhibitors (Figure 2B and supplemental Figure 4C).
FICZ-induced enhancement of mediator release is calcium and ROS dependent. BMMCs were sensitized with E-C1 ± 1 nM FICZ or equal amount of vehicle as a control (Con) for 16 hours and then stimulated with 10 μg/mL OVA. The release of (A) Hex and (B) LTC4 in the absence or presence of a calcium blocker (2-APB) and an antioxidant (N-acetyl cysteine) was added 30 minutes before OVA stimulation. Data are representative of 3 independent experiments. (C) FICZ enhances oxidation of a PTP (SHP-2) in mast cells. BMMCs were sensitized with E-C1 ± 1 nM FICZ or an equal amount of vehicle as a control for 16 hours and then stimulated with 10 μg/mL OVA for the indicated time. The cells were lysed, and then SHP-1 and SHP-2 were immunoprecipitated (IP). The immunoprecipitates were subjected to alkylation by IAA, followed by reduction of the reversibly oxidized PTPs with dithiothreitol, and by final oxidation with pervanadate. After size fractionation by SDS-PAGE, the samples were subjected to immunoblotting (IB) with oxPTP mAbs and after stripping, with the respective Abs as loading controls. Data are representative of 3 independent experiments. (D) BMMCs were treated as above. OVA-stimulated cells were lysed and SHP-2 was immunoprecipitated, and the relative activity of PTPs was determined by measuring the levels of FDP fluorescence (RFU). *P < .05. Data are representative of 3 independent experiments.
FICZ-induced enhancement of mediator release is calcium and ROS dependent. BMMCs were sensitized with E-C1 ± 1 nM FICZ or equal amount of vehicle as a control (Con) for 16 hours and then stimulated with 10 μg/mL OVA. The release of (A) Hex and (B) LTC4 in the absence or presence of a calcium blocker (2-APB) and an antioxidant (N-acetyl cysteine) was added 30 minutes before OVA stimulation. Data are representative of 3 independent experiments. (C) FICZ enhances oxidation of a PTP (SHP-2) in mast cells. BMMCs were sensitized with E-C1 ± 1 nM FICZ or an equal amount of vehicle as a control for 16 hours and then stimulated with 10 μg/mL OVA for the indicated time. The cells were lysed, and then SHP-1 and SHP-2 were immunoprecipitated (IP). The immunoprecipitates were subjected to alkylation by IAA, followed by reduction of the reversibly oxidized PTPs with dithiothreitol, and by final oxidation with pervanadate. After size fractionation by SDS-PAGE, the samples were subjected to immunoblotting (IB) with oxPTP mAbs and after stripping, with the respective Abs as loading controls. Data are representative of 3 independent experiments. (D) BMMCs were treated as above. OVA-stimulated cells were lysed and SHP-2 was immunoprecipitated, and the relative activity of PTPs was determined by measuring the levels of FDP fluorescence (RFU). *P < .05. Data are representative of 3 independent experiments.
FICZ enhances oxidation of a PTP, SHP-2, in mast cells
The consequences of increased levels of ROS on mast cell activation were then analyzed. One of the potential regulatory targets is the family of PTPs; recently, reversible inhibitory oxidation of the active-site cysteine on PTPs by ROS has emerged as an important regulatory mechanism.18-22 To investigate the potential effects of FICZ on PTP oxidation via the generation of ROS, the levels of oxidized SHP-1 and SHP-2, 2 critical PTPs controlling mast cell signaling,23 were examined.
The results showed that although SHP-2 showed no apparent oxidation in quiescent cells, a time-dependent increase in the levels of oxidized SHP-2, but not SHP-1, was noted in FICZ-treated cells after the stimulation of the cells with OVA (Figure 2C). Functionally, as seen in Figure 2D, while in the absence of FICZ the enzymatic activity of SHP-2 was reduced over time after stimulation of the cells with OVA, the levels of SHP-2 phosphatase activity were further reduced in cells treated with FICZ. Moreover, when the total PTPs were analyzed, a similar increase in the level of oxidation was found in mast cells treated with FICZ. As shown in supplemental Figure 5A, whereas no apparent oxidized PTPs were found in the IgE-sensitized cells without antigen stimulation, several reactive proteins were detected in the OVA-stimulated cells. Significantly, a notable enhancement of oxidation occurred in FICZ-treated cells before OVA challenge, which was more prominent after OVA stimulation and concomitant with reduced levels of phosphatase activity (supplemental Figure 5B).
AhR-null mast cells are refractory to antigen stimulation and deficient at the site of mucosa
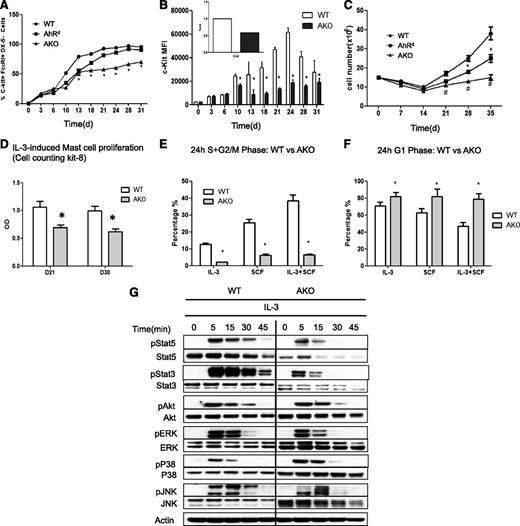
To examine the potential endogenous activity of AhR, the functional characteristics of mast cells from AKO mice and a congenic strain carrying the low-affinity mutant AhRd allele were examined. The growth and differentiation of mast cells (c-kit+FcεRI+CD49−) from the bone marrow cultures were kinetically analyzed during the 4-week culture time period. To our surprise, when BMMCs from AKO and AhRd mice were analyzed, a significant reduction in cell growth and differentiation was noted in AKO mast cells. As shown in Figure 3A, while, as expected, bone marrow cells from the wild-type (WT) mice differentiated into mast cells normally, reaching >95% in purity 4 weeks after the initiation of culture, the percentage of the mast cell population from AKO mice was significantly lower than that from WT mice (Figure 3A and supplemental Figure 6A). Also, during the differentiation/maturation process, there was no apparent difference in the level of the α subunit of the high-affinity IgE receptor complex, FcεRIα (supplemental Figure 6A), but the relative level of c-kit, a known target for AhR-mediated transcription,8 was significantly lower in AKO mast cells compared with WT cells (Figure 3B and supplemental Figure 6B). The same difference at the level of steady-state c-kit gene expression was also found between the 2 cell types (Figure 3B inset).
Growth defect in AKO and AhRd mast cells in response to IL-3. (A) The percentages of c-Kit+FcεRI+CD49b- mast cells. Bone marrow cells were cultured in 30% WEHI-3-conditioned medium, and at indicated time points, aliquots of cultured cells from WT, AhRd, and AKO mice were stained for c-Kit, FcεRI, and CD49b and analyzed by flow cytometry. *P < .05 (WT vs AKO). Data are representative of 3 independent experiments. (B) The relative level of c-kit analyzed by flow cytometry and measured as mean fluorescence intensity (MFI). Inset: c-kit expression detected by real-time reverse-transcription polymerase chain reaction from D31 mast cells.*P < .05. Data are representative of 3 independent experiments. (C) To determine the cell numbers, media were changed weekly and the numbers of suspension cells excluding trypan blue were recorded over 5 weeks. *P < .05 (WT vs AhRd); #P < .05 (WT vs AKO). Data are representative of 3 independent experiments. (D) Mast cells were negatively selected on day 21 (D21) and day 30 (D30) after the initiation of the culture and stimulated with 5 ng/mL IL-3 for 3 days and the cell proliferation was evaluated with Cell counting kit-8. *P < .05. Data are representative of 3 independent experiments. BMMCs were first synchronized at G0/G1 in media devoid of cytokines for 16 hours and then stimulated with IL-3 (5 ng/mL) and/or SCF (20 ng/mL). The percentages of the cell population at (E) the S+G2/M and (F) G1 phases were determined at the 24-hour time point. *P < .05. Data are representative of 3 independent experiments. (G) Analysis of IL-3–induced signaling. Mature BMMCs from WT or AKO mice were first cultured in the cytokine-free media for 6 hours and then stimulated with 5 ng/mL IL-3 for different time points, followed by western blotting analysis of activation of STATs, AKT, and MAPKs in cell lysates by the use of the respective anti-phospho or total protein antibodies. Data are representative of 3 independent experiments.
Growth defect in AKO and AhRd mast cells in response to IL-3. (A) The percentages of c-Kit+FcεRI+CD49b- mast cells. Bone marrow cells were cultured in 30% WEHI-3-conditioned medium, and at indicated time points, aliquots of cultured cells from WT, AhRd, and AKO mice were stained for c-Kit, FcεRI, and CD49b and analyzed by flow cytometry. *P < .05 (WT vs AKO). Data are representative of 3 independent experiments. (B) The relative level of c-kit analyzed by flow cytometry and measured as mean fluorescence intensity (MFI). Inset: c-kit expression detected by real-time reverse-transcription polymerase chain reaction from D31 mast cells.*P < .05. Data are representative of 3 independent experiments. (C) To determine the cell numbers, media were changed weekly and the numbers of suspension cells excluding trypan blue were recorded over 5 weeks. *P < .05 (WT vs AhRd); #P < .05 (WT vs AKO). Data are representative of 3 independent experiments. (D) Mast cells were negatively selected on day 21 (D21) and day 30 (D30) after the initiation of the culture and stimulated with 5 ng/mL IL-3 for 3 days and the cell proliferation was evaluated with Cell counting kit-8. *P < .05. Data are representative of 3 independent experiments. BMMCs were first synchronized at G0/G1 in media devoid of cytokines for 16 hours and then stimulated with IL-3 (5 ng/mL) and/or SCF (20 ng/mL). The percentages of the cell population at (E) the S+G2/M and (F) G1 phases were determined at the 24-hour time point. *P < .05. Data are representative of 3 independent experiments. (G) Analysis of IL-3–induced signaling. Mature BMMCs from WT or AKO mice were first cultured in the cytokine-free media for 6 hours and then stimulated with 5 ng/mL IL-3 for different time points, followed by western blotting analysis of activation of STATs, AKT, and MAPKs in cell lysates by the use of the respective anti-phospho or total protein antibodies. Data are representative of 3 independent experiments.
Moreover, it was noted that in the first 2 weeks of culture, the cell numbers remained similar among AhRd, AKO, and WT mast cells (Figure 3C). However, after 2 weeks, the growth of AhRd and AKO mast cells was significantly retarded as compared with WT mast cells. To directly assess whether there was a difference in mast cell proliferation without interference from other cell types, mast cells were negatively selected on day 21 and day 30 after initiation of the culture and stimulated with IL-3 for 3 days, and the number of viable cells was evaluated. As seen in Figure 3D, mature AKO mast cells proliferated poorly in response to IL-3 stimulation as compared with WT cells.
Further, as noted in IL-3– and SCF-induced cell-cycle analysis (Figure 3E), while WT mast cells exhibited a consistent increase in the S+G2/M-phase progression after 24 hours in cultures containing IL-3, SCF, or a combination of IL-3 and SCF, the majority of the AKO mast cells were arrested at the G1 phase at the 24-hour time point and were not able to advance into the S+G2/M phase (Figure 3F). To address the proliferation difference between WT and AKO mast cells, 3 major pathways,24 including mitogen-activated protein kinase (MAPK), Jak-stat, and PI3-Akt, were examined after IL-3 stimulation. In fact, there was a significant reduction in the levels of total cytosolic STAT3 and STAT5 in both AKO (Figure 3G) and AhRd (supplemental Figure 7A) mast cells, although the STAT proteins in AKO and AhRd mast cells showed similar kinetic activation to that seen in WT mast cells following the challenge with IL-3. However, with real-time PCR we could not find significant difference in the levels of STAT3 and STAT5 transcripts between WT and AKO mast cells. This may be due to posttranslational regulation, since an enhanced level of STAT3 ubiquitination was noted in AKO BMMCs as compared with that in WT cells (supplemental Figure 7B). In contrast, other signaling events, including induction of AKT and MAPKs, appeared to be less affected (Figure 3G). The level of IL-3 receptor expression was similar in all conditions (data not shown). Consistent with the in vitro–derived mast cells, mast cell deficiency was confirmed in various tissues and the peritoneal cavity of AKO mice. For example, immunohistochemistry staining of skin mast cells showed a significant reduction of skin mast cells in AKO mice as compared with WT mice (Figure 4A-C). Similar reduction in mast cell numbers was also found in the peritoneal cavity (Figure 4D and supplemental Figure 8A) and at the site of stomach mucosa in AKO mice (supplemental Figure 8B). These results suggest that AhR is critical in regulating the differentiation and growth of mast cells in vivo and ex vivo.
Immunohistochemical staining of mast cells. Toluidine blue staining (A) and safranin/alcian blue staining (B) of mast cells (indicated by arrows in red) in the back skin from WT and AKO mice at 12 weeks of age (original magnification ×200). Scale bar represents 50 μm. Data are representative of 3 independent experiments. (C) Quantitative analysis of (A) *P < .05, n = 9. (D) Quantitative analysis of peritoneal mast cells by Toluidine blue staining. *P < .05, n = 10. Data are representative of 3 independent experiments.
Immunohistochemical staining of mast cells. Toluidine blue staining (A) and safranin/alcian blue staining (B) of mast cells (indicated by arrows in red) in the back skin from WT and AKO mice at 12 weeks of age (original magnification ×200). Scale bar represents 50 μm. Data are representative of 3 independent experiments. (C) Quantitative analysis of (A) *P < .05, n = 9. (D) Quantitative analysis of peritoneal mast cells by Toluidine blue staining. *P < .05, n = 10. Data are representative of 3 independent experiments.
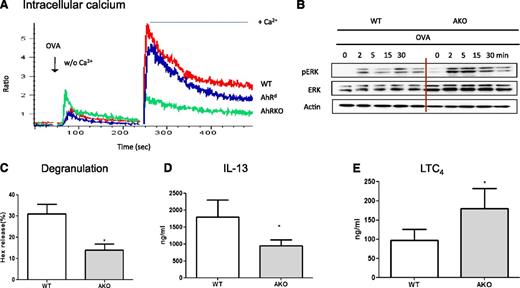
Interestingly, when the levels of IgE/antigen-induced intracellular calcium were measured, AKO mast cells showed an apparent defect in calcium signaling. As noted in Figure 5A, when sensitized mast cells were challenged with OVA in the absence of exogenous Ca2+, AKO mast cells showed elevated and the highest levels of [Ca2+]i as compared with WT and AhRd mast cells. However, when the cells were shifted to calcium-containing buffer 4 minutes later, a significant burst in [Ca2+]i was first noted in WT mast cells and, to a lesser degree, in AhRd mast cells. In contrast, no significant increase in calcium influx was found in AKO mast cells, suggesting the possible involvement of AhR in regulating the calcium mobilization from the intracellular stores and its influx from the extracellular source. Moreover, significantly upregulated levels of ERK kinase and its activation were found in AKO mast cells when compared with WT cells (Figure 5B). Consequently, AKO mast cells demonstrated significantly lower levels of degranulation (Figure 5C) and IL-13 production (Figure 5D); interestingly, the levels of LTC4 release appeared to be significantly increased in AKO mast cells (Figure 5E), consistent with their elevated levels of ERK activation.
AKO mast cells were refractory to IgE/antigen stimulation. (A) Ca2+-mobilization and influx. BMMCs of WT and AKO were sensitized with IgE (E-C1) for 16 hours and then loaded with the Ca2+ indicator Fluo-3-AM and Fura red-AM. Ca2+ release was elicited by stimulation with 10 μg/mL OVA (Ag) first in Ca2+-free conditions, followed by shifting to Ca2+ (2 mM)-containing buffer 4 minutes later. The resulting changes in intracellular calcium were monitored over time. Data are representative of 3 independent experiments. (B) BMMCs were sensitized with E-C1 and then stimulated with 10 μg/mL OVA for the indicated time. Whole-cell lysates were analyzed by western blot. Data are representative of 3 independent experiments. (C) The levels of degranulation, IL-13 (D), and LTC4 (E) in WT or AKO mast cells sensitized with IgE (E-C1) for 16 hours and stimulated with OVA for 30 minutes for degranulation and LTC4 or 6 hours for IL-13 measurement. *P < .05. Data are representative of 5 independent experiments.
AKO mast cells were refractory to IgE/antigen stimulation. (A) Ca2+-mobilization and influx. BMMCs of WT and AKO were sensitized with IgE (E-C1) for 16 hours and then loaded with the Ca2+ indicator Fluo-3-AM and Fura red-AM. Ca2+ release was elicited by stimulation with 10 μg/mL OVA (Ag) first in Ca2+-free conditions, followed by shifting to Ca2+ (2 mM)-containing buffer 4 minutes later. The resulting changes in intracellular calcium were monitored over time. Data are representative of 3 independent experiments. (B) BMMCs were sensitized with E-C1 and then stimulated with 10 μg/mL OVA for the indicated time. Whole-cell lysates were analyzed by western blot. Data are representative of 3 independent experiments. (C) The levels of degranulation, IL-13 (D), and LTC4 (E) in WT or AKO mast cells sensitized with IgE (E-C1) for 16 hours and stimulated with OVA for 30 minutes for degranulation and LTC4 or 6 hours for IL-13 measurement. *P < .05. Data are representative of 5 independent experiments.
AKO mast cells show mitochondrial damage
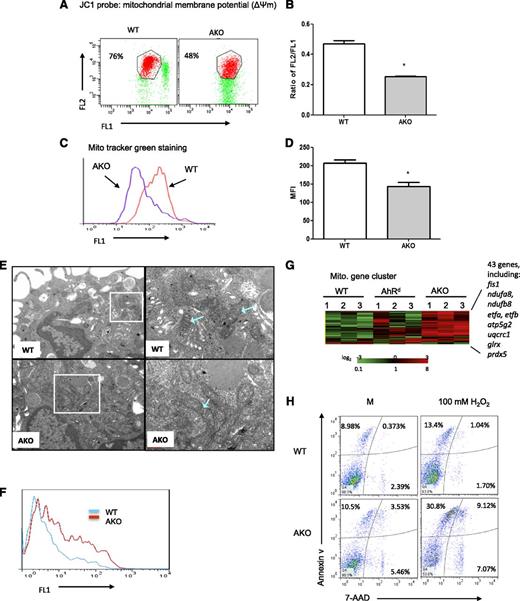
Since store-operated Ca2+ entry depends on mitochondrial Ca2+ uptake and increased mitochondrial depolarization suppresses store-operated Ca2+ influx,25-28 mitochondrial membrane potentials were analyzed by the use of a dual-wavelength fluorescent probe, JC-1, which as a monomer emits green fluorescence and in a reaction driven by the mitochondrial membrane potential converts to a red-fluorescence–emitting dimer. As seen in Figure 6A-B, the ratio of red/green JC-1 fluorescence was significantly lower in AKO mast cells relative to the WT cells, suggesting functional mitochondrial alterations. The mitochondrial mass changes measured by JC-1 were confirmed by fluorescence staining with another mitochondrion-selective dye, MitoTracker green (Figure 6C-D). Furthermore, transmission electron microscopy demonstrated that while in WT mast cells the overall shape of the mitochondria as well as the cristae and dense matrix inside were clearly seen, the mitochondria of AKO mast cells appeared to be obscure, the membranes were not as sharply defined as those seen in WT mast cells, and their inner structure contained only a few, less defined cristae (Figure 6E).
AKO mast cells demonstrate mitochondrial damage. (A) Mitochondrial membrane potential was assessed by JC1 staining. Data are representative of 3 independent experiments. (B) Ratios of JC-1 red versus green fluorescence. (C) Mitochondrial mass was detected by MitoTracker green staining. Data are representative of 3 independent experiments. (D) Numerical data of panel C. Data are representative of 3 independent experiments. (E) Transmission electron microscopy images of WT and AKO mast cells. (F) AKO mast cells showed elevated levels of intracellular ROS. Mast cells were cultured in the cytokine-free medium for 4 hours and then labeled with CM-H2DCFDA, and the levels of ROS were detected by flow cytometry. Data are representative of 3 independent experiments. (G) A heatmap of gene-chip array and gene cluster analyses of WT and AKO mast cells. (H) Analysis of apoptosis in WT and AKO mast cells. Mast cells were cultured in the WEHI-3–conditioned medium for 4 weeks. BMMCs were treated with 100 μM H2O2 overnight or using medium (M) as a control, and cellular apoptosis was detected by the use of annexin V and 7-AAD staining. Data are representative of 3 independent experiments.
AKO mast cells demonstrate mitochondrial damage. (A) Mitochondrial membrane potential was assessed by JC1 staining. Data are representative of 3 independent experiments. (B) Ratios of JC-1 red versus green fluorescence. (C) Mitochondrial mass was detected by MitoTracker green staining. Data are representative of 3 independent experiments. (D) Numerical data of panel C. Data are representative of 3 independent experiments. (E) Transmission electron microscopy images of WT and AKO mast cells. (F) AKO mast cells showed elevated levels of intracellular ROS. Mast cells were cultured in the cytokine-free medium for 4 hours and then labeled with CM-H2DCFDA, and the levels of ROS were detected by flow cytometry. Data are representative of 3 independent experiments. (G) A heatmap of gene-chip array and gene cluster analyses of WT and AKO mast cells. (H) Analysis of apoptosis in WT and AKO mast cells. Mast cells were cultured in the WEHI-3–conditioned medium for 4 weeks. BMMCs were treated with 100 μM H2O2 overnight or using medium (M) as a control, and cellular apoptosis was detected by the use of annexin V and 7-AAD staining. Data are representative of 3 independent experiments.
The changes in mitochondrial membrane potential have been associated with uncoupling of the respiratory chain and an elevated production of ROS and are frequently associated with the initial phases of apoptosis. Indeed, elevated levels of intracellular ROS were found in AKO mast cells (Figure 6F), which were further increased when the cells were treated with 100 μM H2O2 for 1 hour, while in WT cells there was no significant enhancement of ROS (supplemental Figure 9). Moreover, the increased ROS levels in AKO mast cells appeared to be primarily the hydrogen peroxide, peroxynitrite, and/or hydroxyl radical species (supplemental Figure 10A,C), while in WT and AKO mast cells treated with pyocyanin, a general ROS inducer, the generation of superoxide was clearly evident, particularly in AKO mast cells (supplemental Figure 10B,D). Furthermore, gene-clustering analysis showed that notable among genes with upregulated expression in 3 different batches of AKO mast cells were 43 genes in total involved in mitochondrial function (Figure 6G), including genes encoding mitochondrial fission 1 protein (fis1), NADH dehydrogenases (ndufa8 and ndufb8), electron-transfer-flavoprotein, β polypeptide (etfa and etfb), ATP synthase lipid-binding protein (atp5g2), cytochrome b-c1 complex subunit 1 (uqcrc1), and the antioxidants glutaredoxin-1 (glrx) and peroxiredoxin-5 (prdx5).
Analysis of cellular apoptosis by the use of annexin V and 7-AAD staining revealed that AKO mast cells showed an increase in the percentage of apoptotic cells (see Figure 6H for a representation, where annexin V+7-AAD+ cells comprised 9.4% of the WT mast cells versus 14.4% of AKO mast cells). When cells were treated with 100 μM H2O2, there was significantly increased apoptosis (annexin V+7-AAD+ cells, 39.9%) in the AKO mast cell population as compared with WT cells (14.0%; Figure 6H). Further, about 15.7% of the AhRd mast cells stained positive for annexin V (see supplemental Figure 11A for a representation), while only 5.1% of the cells were annexin V positive in WT cells. The enhanced apoptosis of the AhRd mast cell population was consistent with their increased activity of the proapoptotic proteins, caspase-3 and caspase-7 (supplemental Figure 11B-C), as well as a decrease in the level of Bcl-2, an antiapoptotic protein (supplemental Figure 11D). Therefore, these results suggest that AhR may play a significant endogenous role in maintaining normal functioning of mitochondria and preventing cellular apoptosis events.
Discussion
As particularly noted in AKO and, to some extent, AhRd mast cells, the absence of AhR or a drop in its ligand-binding affinity appeared to have a significant impact on the differentiation, growth, and optimal activation of mast cells, suggesting its critical endogenous role. Consequently, any deviation from this controlled homeostatic state may result in a significant change in mitochondrial function and an increase in oxidant burden, which may ultimately lead to apoptosis, mast cell deficiency, and dysregulated mast cell response. Moreover, in WT mast cells, a sequential event can be envisaged upon ligand interaction, where the ligand-AhR axis mediates an enhanced mast cell response, through an increased level of intracellular calcium and oxidative stress response. The increased level of ROS may subsequently trigger reversible oxidation of PTP (particularly SHP-2) leading to enhanced activation of the downstream signaling pathways. Collectively, our results suggest that AhR signaling may play a role in controlling mitochondrial function, which may, in turn, maintain cellular homeostasis. As a corollary, 2 very recent observational studies14,15 suggested a potential role of AhR ligands in regulating mast cell response, although in these studies relatively high concentrations of AhR ligands were used and the mechanism remained unclear. Our results thus provide a much-needed mechanistic link between the AhR-ligand axis and mast cell response.
It is also noted that AKO mast cells showed significant alteration in calcium signaling, in particular the influx of exogenous calcium. It has been shown that coupling of stromal interacting molecule 1 (STIM1) in the endoplasmic reticulum with the plasma membrane calcium channel protein Orai1 mediates calcium entry as a consequence of calcium depletion in the endoplasmic reticulum.29,30 Further, mitochondrial calcium uptake is required for STIM1-Orai1–dependent store-operated calcium influx.25-28 In this study, the levels of STIM1 and Orai1 were similar in AhR-WT and AhR-null mast cells (supplemental Figure 12). It is thus likely that the reduction in mitochondrial membrane potential noted in AKO mast cells may influence its calcium uptake and subsequent activation of calcium entry.
In a growing number of systems, ROS function as signaling molecules to mediate various biological responses,20 through, in part, their ability to induce oxidation of cysteine residues on and subsequent function of proteins, including PTPs.18-21,31-33 Herein, mast cells treated with AhR ligands showed increased oxidation of SHP-2, but not the highly homologous SHP-1, and reduced phosphatase activity. This selective oxidation may be due to different spatial orientation of the active-site cysteines in relation to positively charged residues (arginine) in the active-site pocket of the 2 phosphatases34 or due to the specific subcellular location of individual PTP, where localized production of ROS and oxidation occurs. Of note, SHP-2, but not SHP-1, was found to be located in the mitochondria, which is one of the major ROS-producing organelles.35
AhR is known to be a chemical sensor initiating the detoxification process through its ability to induce both phase I and II enzymes. Disruption of this critical function by competitive exogenous ligands or by mechanisms causing fluctuation in its expression level would, therefore, have a direct impact on the normal functioning of the detoxification process. It is, perhaps, the dysregulated detoxification or increased metabolites thereof that function as the endogenous ligands in enhancing the mast cell response and/or causing the cellular damage.36 Interestingly, our results (supplemental Figure 13A-D) showed that FICZ-induced ROS and FICZ’s enhancing effect could be inhibited, at least in part, by inhibitors for 5-lipoxygenase and COX1, but not COX2, suggesting the possible candidacy of eicosanoid metabolites as the endogenous ligands. As a corollary, recent studies have noted that some of the arachidonic acid metabolites, including lipoxin A4 and PGG2, have been suggested to be ligands for AhR.1,37
It is noted in our study, however, that higher doses of AhR ligands did not show the enhancing effects (inverted U-shaped dose response). This dose response is consistent with previous reports in other experimental systems.16,17 Considering the fact that the affinity of FICZ’s binding to AhR is at picomolar to low-nanomolar range (Kd value, 0.07 nM),38 the low-dose exposure of AhR ligands that we demonstrated in this study (0.1-1 nM FICZ) may be more physiologically relevant.
A variety of environmental risk factors have been suggested to be associated with the occurrence of allergic diseases. Considering the critical role of mast cells in allergic diseases and their strategic location at the site of tissue mucosa where coexposure of allergens and environmental chemicals often occurs, exposure to those factors may thus have significant impact on mast cell function or on that of other cell types. The potential regulatory pathway originated from AhR may thus represent a novel regulatory pathway in mast cell homeostasis and in mucosal innate immunity. The fact that AhR is able to control c-kit expression and the level of STAT-associated signaling may have significant implications for the differentiation of other cell types and for other disease contexts where c-kit and STAT signaling are involved.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (AI052468 and AI073610), the National Health Research Institutes of Taiwan (EOPP10-014 and EOSP07-014), and the National Science Council of Taiwan (NSC 100-3114-Y-043-002/00D1-EODOH01).
Authorship
Contribution: Y.Z. designed and conducted experiments characterizing how AhR ligands regulate mast cell function and its endogenous regulatory role in mast cells, analyzed data, and wrote the manuscript. H.-Y.T. and Y.-M.T. conducted mast cell proliferation, differentiation, and PCA studies. S.-C.H. and P.G. were involved in the gene chip study. H.-W.C. and C.-H.H. conducted transmission electron microscopy and HCMC studies. H.K. and H.-C.T. were involved in the ROS study. B.P. performed cell cultures and edited the manuscript. B.M.V. designed the cell signaling study. S.-K.H. planned, designed, supervised, and coordinated the overall research efforts and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shau-Ku Huang, National Health Research Institutes, 35 Keyan Rd, Zhunan, Miaoli County 35053, Taiwan; e-mail: skhuang@nhri.org.tw; and Johns Hopkins Asthma and Allergy Center, 5501 Hopkins Bayview Circle, Baltimore, MD 21224; e-mail: skhuang@jhmi.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal