Key Points
Purification of staged human erythroblasts should facilitate a comprehensive cellular and molecular characterization of these cell populations.
Quantification of human terminal erythropoiesis in vivo provides a powerful means for studying impaired erythropoiesis in human diseases.
Abstract
Terminal erythroid differentiation starts from morphologically recognizable proerythroblasts that proliferate and differentiate to generate red cells. Although this process has been extensively studied in mice, its characterization in humans is limited. By examining the dynamic changes of expression of membrane proteins during in vitro human terminal erythroid differentiation, we identified band 3 and α4 integrin as optimal surface markers for isolating 5 morphologically distinct populations at successive developmental stages. Functional analysis revealed that these purified cell populations have distinct mitotic capacity. Use of band 3 and α4 integrin enabled us to isolate erythroblasts at specific developmental stages from primary human bone marrow. The ratio of erythroblasts at successive stages followed the predicted 1:2:4:8:16 pattern. In contrast, bone marrows from myelodysplastic syndrome patients exhibited altered terminal erythroid differentiation profiles. Thus, our findings not only provide new insights into the genesis of the red cell membrane during human terminal erythroid differentiation but also offer a means of isolating and quantifying each developmental stage during terminal erythropoiesis in vivo. Our findings should facilitate a comprehensive cellular and molecular characterization of each specific developmental stage of human erythroblasts and should provide a powerful means of identifying stage-specific defects in diseases associated with pathological erythropoiesis.
Introduction
Erythropoiesis is a process by which mature red cells are generated from hematopoietic stem cells. This continuum can be subdivided into 3 stages: early erythropoiesis, terminal erythroid differentiation, and reticulocyte maturation. Early erythropoiesis refers to the process by which multi-potential hematopoietic stem cells proliferate and differentiate into committed erythroid progenitors: erythroid burst-forming unit (BFU-E) and then erythroid colony-forming unit (CFU-E) cells that differentiate into proerythroblasts.1 Terminal erythroid differentiation begins with morphologically recognizable proerythroblasts, which subsequently undergo sequential mitoses to become basophilic, polychromatic, and orthochromatic erythroblasts that enucleate to become reticulocytes. During terminal erythroid differentiation, several pronounced changes occur, including decrease in cell size, increase in hemoglobinization, increased chromatin condensation, and enucleation. In addition, biochemical analysis reveals that terminal differentiation is also accompanied by dramatic changes in the expression, as well as assembly, of membrane proteins.2-7 At the final step of erythropoiesis, multilobular reticulocytes mature into discoid erythrocytes accompanied by loss of intracellular organelles,8-10 loss of surface area,11 decrease in cell volume, and reorganization of membrane and skeletal components.12,13
One unique feature of erythropoiesis is that each cell division is simultaneously coupled with differentiation. For most cell types, each cell division generates 2 daughter cells that are almost identical to the mother cell. However, during erythropoiesis, the daughter cells are structurally and functionally different than the mother cell from which they are derived. Thus, to develop a detailed understanding of erythropoiesis, it is critical to obtain cells at all distinct developmental stages. Using Ter119 as an erythroid lineage marker, in conjunction with CD44, and cell size as differentiation markers, we have recently developed a method for distinguishing unambiguously erythroblasts at each developmental stage during murine erythroid differentiation in bone marrow and spleen.6 Our method enabled isolation of erythroblasts at each stage of development in a much more homogenous state than achieved in earlier work based on expression levels of the transferrin receptor, CD71.14,15 Flygare et al16 isolated mouse BFU-E and CFU-E cells from embryonic day 14.5 to 15.5 fetal liver cells by negative selection for Ter119, B220, Mac-1, CD3, Gr1, Sca-1, CD16/CD32, CD41, and CD34 cells, followed by separation based on the expression levels of CD71.
Although the studies of mouse erythropoiesis are relatively extensive, the studies of human erythropoiesis are more limited. To address this issue, we examined the dynamic changes in expression levels of a large number of red cell membrane proteins during human terminal erythroid differentiation. We found that while the expression of major red cell membrane proteins increased, the expression of most adhesion molecules decreased. Particularly, the expression of GPA and band 3 progressively increased and that of α4 integrin decreased. The use of GPA, band 3, and α4 integrin as surface markers enabled us to develop a means to isolate highly purified populations of erythroblasts at each distinct stage from an erythroid culture system and from primary human bone marrow cells. It also enabled the quantification of in vivo human terminal erythroid differentiation. The ability to isolate and quantitate human erythroblasts at distinct stages of development in vivo should enable us to develop a detailed mechanistic understanding of normal human terminal erythroid differentiation and also define stage-specific defects in disordered erythropoiesis in various diseases, such as thalassemias and bone marrow failure syndromes.
Materials and methods
Antibodies for western blot analysis
Antibodies for flow cytometry
Mouse monoclonal antibodies against the extracellular regions of human band 3, glycophorin C (GPC), Kell, CD59, Rhesus-associated glycoprotein (RhAG), and CD147 were generated in our laboratory. Monoclonal antibody against Rhesus disease (RhD) was kindly provided by Dr. Yves Colin (INSERM, INTS, Paris, France). The previously listed antibodies were purified with Protein G Agarose Fast Flow (Millipore, Billerica, MA) and labeled with DyLight 488 antibody labeling kit (Pierce Biotech, Rockford, IL). Commercial antibodies include: Ag-presenting cell (APC)-conjugated CD44 (eBioscience, San Diego, CA); PE-conjugated CD235a, fluorescein isothiocyanate (FITC)-conjugated CD71, FITC-conjugated CD36, and FITC-conjugated CD47 (BD Pharmingen, Franklin Lakes, NJ); PE-conjugated α5 integrin and PE-conjugated β1 integrin (Millipore); and APC-conjugated α4 integrin (Miltenyi Biotec, Auburn, CA).
Materials for cell culture
Human plasma, recombinant IL-3, and recombinant stem cell factor were obtained from Stem Cell Technologies (Vancouver, Canada). Human recombinant erythropoietin and Iscove's Modified Dulbecco's Medium were obtained from Invitrogen. Holo human transferrin, insulin, and heparin were obtained from Sigma Aldrich (St. Louis, MO). Human AB serum was obtained from Atlanta Biologicals (Lawrenceville, GA). Human cord blood samples were obtained from the New York Blood Center Cord Blood Program. Normal and myelodysplastic syndrome (MDS) human bone marrow samples were obtained from The New York Presbyterian Cornell Hospital and Myelodysplastic Syndrome Center, Columbia University Medical Center, New York, New York, respectively, according to the institutional review board approval and in accordance with the Declaration of Helsinki. (The institutional review board approval number is 1204012332.)
Purification and culture of CD34+ cells
CD34+ cells were purified from cord blood by positive selection using the magnetic-activated cell sorting magnetic beads system, according to the manufacturer’s instructions. The purity of isolated CD34+ cells was 95% to 98%. The cell culture procedure was comprised of 3 phases. Composition of the base culture medium was Iscove's Modified Dulbecco's Medium, 2% human peripheral blood plasma, 3% human AB serum, 200 μg/mL Holo-human transferrin, 3 IU/mL heparin, and 10 μg/mL insulin. In the first phase (day 0 to day 6), CD34+ cells at a concentration of 105/mL were cultured in the presence of 10 ng/mL stem cell factor, 1 ng/mL IL-3, and 3 IU/mL erythropoietin. In the second phase (day 7 to day 11), IL-3 was omitted from the culture medium. In the third phase that lasted until day 21, the cell concentration was adjusted to 106/mL on day 11 and to 5 × 106/mL on day 15, respectively, the medium for this phase was the base medium plus 3 IU/mL erythropoietin, and the concentration of transferrin was adjusted to 1 mg/mL. The cells were cultured at 37°C in the presence of 5% CO2.
Western blot analysis
Whole-cell lysates of cultured cells were prepared with radiommunoprecipitation assay buffer (150 mM NaCl, 1.0% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 1 mM EDTA, and 50 mM Tris HCl, pH 8.0) in the presence of protease inhibitor cocktails (Sigma). Protein concentration was measured using a Pierce BCA protein assay kit from Thermo Scientific. Protein (30 μg) was run on a 10% sodium dodecyl sulfate/polyacrylamide gel electrophoresis gel, and western blot analysis was performed as previously described.6,13,17
Flow cytometry analysis
Cells taken from the culture every day (on day 6 to day 20) were analyzed for surface expression of glycophorin A (GPA), GPC, band 3, RhAG, Kell, CD59, CD44, α4 integrin, β1 integrin, α5 integrin, CD71, CD147, CD47, RhD, and CD36. For this, 0.1 × 106 cells were suspended in 20 μL phosphate-buffered saline (PBS) supplemented with 0.5% bovine serum albumin (BSA). After blocking with 0.4% human AB serum for 10 minutes on ice, the cells were stained with fluorochrome-conjugated antibodies for 15 minutes on ice. Cells were washed once with PBS 0.5% BSA before analysis. All the reactions were performed under conditions of antibody saturation. Cells stained with the isotype control antibody were used as a negative control. The samples were analyzed within 1 hour after staining by using BD FACSDiva software and FlowJo software on a FACSCanto flow cytometer.
Fluorescence-activated cell sorting of cultured cells
Cells were washed once with PBS-0.5%BSA, suspended at a concentration of 1 × 106 in PBS 0.5% BSA and blocked with 0.4% human AB serum. The cells were stained with 1 μg/106 cells DyLight 488-conjugated band 3, 2 × 10−4 μg/106 cells phycoerythrin-conjugated GPA, 6 μL APC-conjugated α4 integrin/106cells, 2μl/106cells APC-Cy7-conjugated CD45, APC-Cy7-conjugated CD11b, and incubated on ice for 15 minutes. After washing with PBS 0.5% BSA, the cells were resuspended at the density of 30 × 106/mL in PBS 0.5% BSA. The cells are sorted on a MoFlo high-speed cell sorter (Beckman-Coulter, Brea, CA).
Culture of sorted erythroblasts
An aliquot of 0.4 × 106 sorted erythroblasts at each distinct developmental stage was cultured in 4 mL of the second phase culture media (for proerythroblast, early basophilic, and late basophilic erythroblasts) or the third phase culture media (for polychromatic and orthochromatic erythroblasts). The cell numbers were counted every day.
Flow cytometric analysis and fluorescence-activated cell sorting of primary human bone marrow cells
Bone marrow cells from normal donors or MDS patients were first diluted with an equal volume of PBS containing 0.5% BSA and were subsequently separated on a Ficoll density gradient at 400 g for 30 minutes at room temperature. The mononuclear cells at the interface were collected and washed once at 300 g for 10 minutes and twice at 200 g for 10 minutes. The cells were incubated with CD45 microbeads for negative selection according to the manufacturer’s instructions. The CD45- cells were stained, analyzed, and sorted as previously described.
Cytospin preparation
The 105 cells in 300 μL were used to prepare cytospin preparations on coated slides, using the Thermo Scientific Shandon 4 Cytospin. The slides were stained with May-Grunwald (Sigma MG500) solution for 5 minutes, rinsed in 40 mM Tris buffer (pH 7.2) for 90 seconds, and subsequently stained with Giemsa solution (Sigma GS500) for 15 minutes. The cells were imaged using a Leica DM2000 inverted microscope.
Results
Expression of skeletal and membrane proteins as assessed by western blot analysis
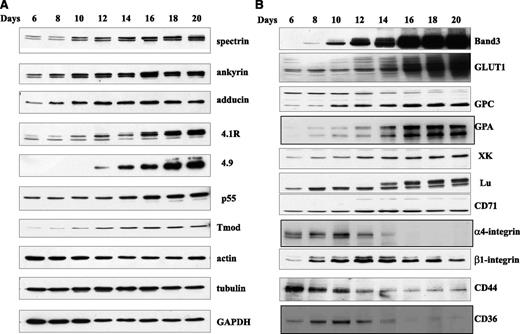
First we examined the total cellular expression of skeletal and membrane proteins by western blot analysis in differentiating erythroid cells collected every other day starting from day 6 of the 20 days of culture. In this culture system, terminal erythroid differentiation approximately starts on day 6 when GPA positive proerythroblasts begin to appear.18 As shown in supplementary Figure 1, the proerythroblasts progressively differentiate to basophilic erythroblasts, polychromatic erythroblasts, orthochromatic erythroblasts, and reticulocytes over subsequent days in culture. The expression levels of 10 skeletal proteins are shown in Figure 1A, which reveals that the amounts of α-spectrin, β-spectrin, ankyrin, 4.1R, p55, adducin, dematin, and tropomodulin increased during terminal differentiation, whereas the quantity of actin decreased in late-stage erythroblasts compared with early stage erythroblasts. In contrast, the expression of tubulin remained relatively constant. Several distinct patterns of protein expression could be distinguished in our analysis of 11 transmembrane proteins (Figure 1B). The first consisted of a low level of expression in proerythroblasts with progressive and dramatic increase in late-stage erythroblasts. Two proteins, band 3 and GLUT1, display this pattern. The second pattern was characterized by progressive increase starting with lower level expression in early stages and continual increase as terminal differentiation progresses. GPC, GPA, XK, Lu, and CD71 belong to this group. The third pattern consisted of a high level expression in proerythroblasts with progressive decrease in late-stage erythroblasts. The adhesion molecules α4 integrin, β1 integrin, CD44, and thrombospondin receptor CD36 belong to this group.
Immunoblots of membrane proteins of erythroblasts at different stages of human terminal erythroid differentiation. Blots of SDS-PAGE of total cellular protein prepared from erythroblasts cultured for different days were probed with antibodies against the indicated proteins. (A) Skeletal proteins and (B) transmembrane proteins. GADPH, glyceraldehyde-3-phosphate dehydrogenase.
Immunoblots of membrane proteins of erythroblasts at different stages of human terminal erythroid differentiation. Blots of SDS-PAGE of total cellular protein prepared from erythroblasts cultured for different days were probed with antibodies against the indicated proteins. (A) Skeletal proteins and (B) transmembrane proteins. GADPH, glyceraldehyde-3-phosphate dehydrogenase.
Surface expression of transmembrane proteins as assessed by flow cytometry
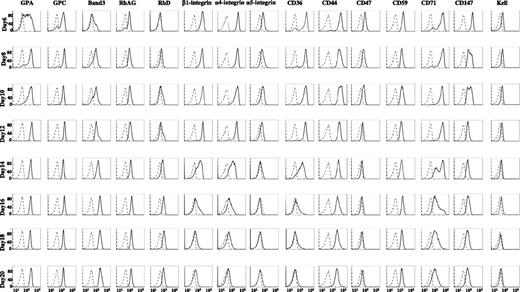
A major objective of the present study was to define surface markers that could be used to isolate human erythroblasts at distinct stages of development. Therefore, after determining total protein expression levels in cells by western blot analysis, we analyzed the expression levels of 15 membrane proteins on cell surface of intact cells by flow cytometry (Figure 2). We observed 2 distinct patterns: the first pattern showed increased expression levels during differentiation, whereas the second pattern revealed decreased expression during differentiation. The group of proteins depicting an increased expression pattern consisted of major red cell membrane molecules, including GPA, GPC, band 3, RhAG, and RhD. Within this group, GPA, GPC, and RhAG appeared on day 6, whereas band 3 and Rh appeared later. Moreover, the expression of band 3 showed the most dramatic and progressive increase over time. It should be noted that expression pattern of GLUT1 is very similar to that of band 3 (data not shown). The group of proteins demonstrating the second pattern of decreased expression included adhesion molecules α4 integrin, α5 integrin, β1 integrin, CD44, and other molecules, such as CD36, CD47, CD59, CD71, CD147, and Kell. Within this group, α4 integrin was highly expressed on day 6 and erythroblasts stayed high until day 12, after which time its expression level progressively decreased.
Flow cytometric analysis of expression of membrane proteins at cell surface at different stages of human terminal erythroid differentiation. The erythroblasts cultured for different days were stained with antibodies against the indicated proteins. The ordinate measures the number of cells displaying the fluorescent intensity given by the abscissa. Note progressive and dramatically increased expression of band 3 throughout terminal erythroid differentiation and decreased expression of α4 integrin during the late stage of terminal erythroid differentiation.
Flow cytometric analysis of expression of membrane proteins at cell surface at different stages of human terminal erythroid differentiation. The erythroblasts cultured for different days were stained with antibodies against the indicated proteins. The ordinate measures the number of cells displaying the fluorescent intensity given by the abscissa. Note progressive and dramatically increased expression of band 3 throughout terminal erythroid differentiation and decreased expression of α4 integrin during the late stage of terminal erythroid differentiation.
Analysis of in vitro human terminal erythroid differentiation using α4 integrin and band 3 as surface markers
It is well recognized that GPA is an erythroid specific marker that starts to appear on the surface of proerythroblasts.19,20 The current findings that surface expression of α4 integrin decreased progressively during late stages of human terminal erythroid differentiation while surface expression of band 3 progressively increased during the entire span of terminal differentiation suggests that changes in the surface expression of these 2 proteins, in conjunction with GPA as a lineage marker, could be valuable for monitoring the progression of human terminal differentiation and for distinguishing erythroblasts at distinct stages of differentiation. To test this hypothesis, we stained the cells every other day with antibodies against GPA, band 3, and α4 integrin. The pattern of expression levels of band 3 vs α4 integrin of the GPA-positive cells revealed distinct and progressive temporal changes (Figure 3). On day 6, all of the cells expressed high levels of α4 integrin with low or no expression of band 3. In contrast, on day 20, all of the cells expressed high levels of band 3 and were α4 integrin negative. Between day 6 and day 20, there was a decrease in the α4 integrinhiband 3low population, with progressive increase in the α4 integrinlowband 3hi population.
Flow cytometric analysis of in vitro differentiated human erythroid cells. The in vitro cultured erythroblasts at different days were stained with GPA, α4 integrin, and band 3. The plots of α4 integrin vs band 3 of all TER-positive cells are shown. Note the progressive change of α4 integrinhiband3neg population to α4 integrinnegband3hi population during terminal erythroid differentiation.
Flow cytometric analysis of in vitro differentiated human erythroid cells. The in vitro cultured erythroblasts at different days were stained with GPA, α4 integrin, and band 3. The plots of α4 integrin vs band 3 of all TER-positive cells are shown. Note the progressive change of α4 integrinhiband3neg population to α4 integrinnegband3hi population during terminal erythroid differentiation.
Sorting of erythroblasts at distinct stages of development from cultured CD34+ cells
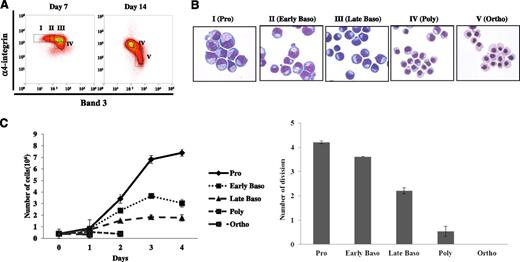
Having shown that a combination of GPA, band 3, and α4 integrin expression levels can be used for distinguishing human erythroblasts at distinct development stages, we then attempted to determine if these markers can be used to obtain highly enriched populations of differentiating erythroblasts by fluorescence-activated cell sorting. We chose cells cultured for 7 and 14 days for sorting early and late stage erythroblasts, respectively. On day 7, 4 of the populations were separated according to the expression levels of α4 integrin and band 3 (Figure 4A, left panel). They are designated as α4 integrinhiband3neg (I), α4 integrinhiband3low (II), α4 integrinhiband 3med (III), and α4 integrinmedband3med (IV). On day 14, 2 major populations can be seen: α4 integrinmedband3med population (IV), similar to that seen on day 7, and another distinct population, α4 integrinlowband 3hi (V) (Figure 4A, right panel). The stained cytospin preparations of these 5 sorted populations revealed that cells from I to V morphologically represented proerythroblasts, early basophilc, late basophilic, polychromatic, and orthochromatic erythroblasts, respectively (Figure 4B).
Isolation and characterization of human erythroblasts at distinct stages of development by cell sorting using GPA, band 3, and α4 integrin as surface markers. (A) The in vitro cultured day 7 or day 14 erythroblasts were stained with GPA, α4 integrin, and band 3. The expression levels of α4 integrin of all GPA+ cells were plotted again the expression levels of band 3. The data are displayed using both contour and density plots. Band 3 negative cells are gated as population I. Population III is represented by the cluster expressing medium level of band 3 and high level of α4 integrin. The region between I and III is gated as population II. Two distinct populations (IV and V) are clearly separated on cells cultured for 14 days. (B) Representative images of erythroblast morphology on stained cytospins from the 6 distinct regions shown in Figure 4A. Pro, proerythroblast; early baso, early basophilic erythroblast; late baso, late basophilic erythroblast; poly, polychromatic erythroblast; ortho, orthochromatic erythroblast. (C) Mitotic ability of purified staged human erythroblasts. Left panel: representative growth curves of staged human erythroblasts. Right panel: number of cell divisions of staged human erythroblasts. Data shown are from 4 independent experiments.
Isolation and characterization of human erythroblasts at distinct stages of development by cell sorting using GPA, band 3, and α4 integrin as surface markers. (A) The in vitro cultured day 7 or day 14 erythroblasts were stained with GPA, α4 integrin, and band 3. The expression levels of α4 integrin of all GPA+ cells were plotted again the expression levels of band 3. The data are displayed using both contour and density plots. Band 3 negative cells are gated as population I. Population III is represented by the cluster expressing medium level of band 3 and high level of α4 integrin. The region between I and III is gated as population II. Two distinct populations (IV and V) are clearly separated on cells cultured for 14 days. (B) Representative images of erythroblast morphology on stained cytospins from the 6 distinct regions shown in Figure 4A. Pro, proerythroblast; early baso, early basophilic erythroblast; late baso, late basophilic erythroblast; poly, polychromatic erythroblast; ortho, orthochromatic erythroblast. (C) Mitotic ability of purified staged human erythroblasts. Left panel: representative growth curves of staged human erythroblasts. Right panel: number of cell divisions of staged human erythroblasts. Data shown are from 4 independent experiments.
Mitotic capacity of erythroblast populations at distinct development stages
It is well established in mice that proerythroblasts undergo 3 mitoses to generate, sequentially 2 basophilic erythroblasts, 4 polychromatic erythroblasts, and 8 orthochromatic erythroblasts.21 It has been suggested that human erythroblasts undergo an additional mitosis.22 To explore this issue, we cultured sorted pure erythroblasts at each of the 5 developmental stages and examined their ability to proliferate. The representative growth curves of these cells showed that while proerythroblasts continued to proliferate for 4 days and expanded approximately 18 times during this period (from 0.4 × 106 to 7.4 × 106), orthochromatic erythroblasts were not able to divide at all (Figure 4C). Quantitative analysis of the proliferation from 4 independent experiments demonstrated that proerythroblasts, early basophilc, late basophilic, polychromatic, and orthochromatic erythroblasts underwent 4, 3, 2, 1, and 0 mitosis, respectively (Figure 4D). Thus, these results further validate the identity of our purified populations of erythroblasts at each distinct stage of development at the functional level.
Analysis of terminal erythroid differentiation and sorting of erythroblasts from primary human bone marrow cells
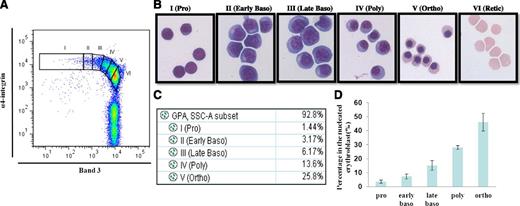
Having established that the combination of GPA, band 3, and α4 integrin can be used as surface markers for isolating distinct stages of erythroblasts from an in vitro CD34+ culture system, we then used these markers to examine the terminal erythroid differentiation of primary human bone marrow cells. The plot of band 3 vs α4 integrin of GPA positive cells revealed 2 clearly separated populations: an α4 integrin+ population that contained nucleated erythroid cells, and an α4 integrin- population that contained enucleated erythroid cells (Figure 5A). Six populations were gated on the α4 integrin+ population based on the expression levels of α4 integrin and band 3. The gated cell populations were sorted using fluorescence-activated cell sorter. The stained cytospins of the sorted cell populations revealed that populations I, II, III, IV, V, and VI represented proerythroblasts, early basophilic, late basophilic, polychromatic, and orthochromatic erythroblasts and reticulocytes, respectively (Figure 5B). Importantly, Figure 5C reveals that proerythroblasts, early basophilic, late basophilic, polychromatic, and orthochromatic erythroblasts account for 1.44%, 3.17%, 6.17%, 13.6%, and 25.8% of the total GPA-positive cells, respectively, demonstrating the doubling of the cell populations after each mitosis. The quantification of the proportion of cells from 6 normal controls after normalization based on total nucleated erythroid cells as 100%, is shown in Figure 5D indicating that proerythroblasts, early basophilic, late basophilic, polychromatic, and orthochromatic erythroblasts account for 3.6%, 7.2%, 15.2%, 28%, and 46% of the total nucleated erythroblasts, respectively, validating the doubling of cells from 1 stage to the next.
Flow cytometry analysis and isolation of primary human bone marrow erythroblasts. CD45- cells isolated from primary human bone marrow were stained with GPA, α4 integrin, and band 3. (A) Plot of band 3 vs α4 integrin of GPA+ cells. (B) Representative images of the sorted cells gated in A. (C) Proportions of distinct stages of erythroblasts in bone marrow shown in A. (D) Quantitation of the proportion of cells at each distinct stage of maturation after normalization based on total nucleated erythroid cells as 100% (N = 7).
Flow cytometry analysis and isolation of primary human bone marrow erythroblasts. CD45- cells isolated from primary human bone marrow were stained with GPA, α4 integrin, and band 3. (A) Plot of band 3 vs α4 integrin of GPA+ cells. (B) Representative images of the sorted cells gated in A. (C) Proportions of distinct stages of erythroblasts in bone marrow shown in A. (D) Quantitation of the proportion of cells at each distinct stage of maturation after normalization based on total nucleated erythroid cells as 100% (N = 7).
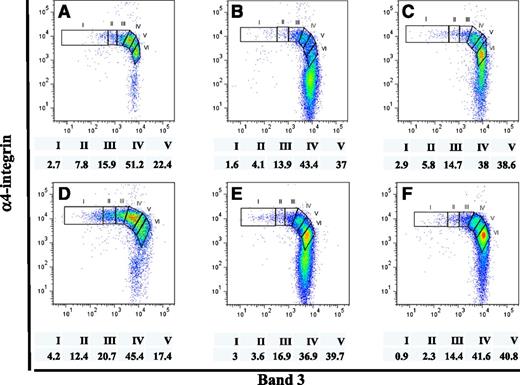
Altered terminal erythroid differentiation profiles of primary human bone marrow cells from patients with MDS
MDS is a group of clonal disorders of the hematopoietic stem cells characterized by ineffective erythropoiesis.23 Having established the method to quantitatively monitor terminal erythroid differentiation in primary normal human bone marrow, we explored whether the application of this method can be used to document disordered erythropoiesis in bone marrow of MDS patients. The terminal erythroid differentiation profiles of 6 MDS patients, 1 from each subtype of MDS, are shown in Figure 6. Strikingly, in all cases, the proportion of erythroblasts at successive stages of differentiation did not follow the predicated doubling pattern that is observed in normal bone marrow. These results indicate that the approach we have developed indeed can be used to monitor abnormalities in the progression of terminal differentiation in MDS and should enable the defining of stage specific defects in erythropoiesis in various subtypes of MDS, as well as responses to therapies.
Terminal erythropoiesis profiles of primary human bone marrow cells from MDS patients. CD45- cells isolated from primary human bone marrow of 6 MDS patients, each with a different MDS subtype, were stained and analyzed, as described in Figure 5. Proportions of distinct stages of erythroblasts, after normalization based on total nucleated erythroid cells as 100%, are indicated. Panels A, B, C, D, E, and F show the terminal erythropoiesis profile of the MDS subtype refractory anemia, refractory anemia with ringed sideroblasts, refractory cytopenia with multilineage dysplasia, refractory cytopenia with multilineage dysplasia and ringed sideroblasts, refractory anemia with excess blasts-1, and refractory anemia with excess blasts-2, respectively. (I) proerythroblasts; (II) early basophilic erythroblasts; (III) late basophilic erythroblasts; (IV) polychromatic erythroblasts; and (V) orthchromatic erythroblasts.
Terminal erythropoiesis profiles of primary human bone marrow cells from MDS patients. CD45- cells isolated from primary human bone marrow of 6 MDS patients, each with a different MDS subtype, were stained and analyzed, as described in Figure 5. Proportions of distinct stages of erythroblasts, after normalization based on total nucleated erythroid cells as 100%, are indicated. Panels A, B, C, D, E, and F show the terminal erythropoiesis profile of the MDS subtype refractory anemia, refractory anemia with ringed sideroblasts, refractory cytopenia with multilineage dysplasia, refractory cytopenia with multilineage dysplasia and ringed sideroblasts, refractory anemia with excess blasts-1, and refractory anemia with excess blasts-2, respectively. (I) proerythroblasts; (II) early basophilic erythroblasts; (III) late basophilic erythroblasts; (IV) polychromatic erythroblasts; and (V) orthchromatic erythroblasts.
Discussion
In the present study, we first performed a comprehensive analysis of changes in the expression of 27 red cell membrane proteins during human terminal erythroid differentiation by both western blot analysis and flow cytometry. Our findings provide insights into the ordered synthesis and assembly of human red cell membrane proteins. Previously, we documented that all of the skeletal proteins of the mature red blood cell, except actin, accrued progressively during murine terminal erythroid differentiation.6 Here we show that similar changes occur during human terminal erythroid differentiation. These findings suggest that the accumulation of mature red cell skeletal proteins during the late stages of erythropoiesis is a conserved feature of both human and murine erythropoiesis.
It should be noted, however, that some differences exist between human and mouse in terms of the expression of membrane proteins. Although it has been shown that the expression of adhesion molecule Lu decreased during murine terminal erythroid differentiation,6 we, as well as others, have shown that its expression increased during human terminal erythroid differentiation.24 Furthermore, while we observed a 30-fold decrease in CD44 expression from the proerythroblasts to the orthochromatic erythroblast stage in the murine system,6 we found only a threefold decrease in the human system over the same differentiation period. These findings clearly suggest that the roles played by these adhesion molecules may be different between human and mouse. They further suggest that studies using various genetically modified mice cannot in all cases be directly extrapolated to human disease. Indeed comparative analysis of the human and murine transcriptomes during terminal erythroid differentiation revealed differences in the expression of many genes between human and mouse. For example, while the mitogen-activated protein kinase pathway is up-regulated during human terminal erythroid differentiation, it is down-regulated during murine terminal erythroid differentiation (our unpublished data).
A major outcome of the systematic examination of surface expression of transmembrane proteins during human terminal erythroid differentiation is the rational choice of surface markers that enabled us to develop a strategy to isolate human erythroblasts at distinct developmental stages. It is important to note that the purified staged erythroblasts not only morphologically resembled erythroblasts at distinct differentiation stages, but that their identities were further supported functionally by documenting their expected proliferative and mitotic capacity.
In vitro human erythroid culture systems have been widely used to study normal erythropoiesis,25,26 as well as diseases associated with altered erythropoiesis such as thalassemia,27 MDS,28 and malaria infection.29 To date, all of these studies have used GPA or/and CD71 as indicators of erythroid differentiation stages. However, because temporal changes in the surface expression of GPA and CD71 are not changed substantially during human terminal erythroid differentiation, the use of these markers does not enable optimal separation of human erythroblasts at distinct stages. In contrast, our current data unquestionably demonstrate that the combined use of band 3, whose surface expression increases, and α4 integrin expression that decreases, enable a clear distinction of erythroblasts at distinct stages of development. Thus, our newly established method would allow monitoring in vitro human terminal erythropoiesis in a stage-specific manner.
Another notable contribution of the present study is the ability to obtain staged human erythroblasts in large quantity and high purity. This is important because it has many potential applications. First, it should enable a detailed molecular characterization of these distinct populations that will in turn provide insights into the mechanisms of erythropoiesis. For example, we have performed genome-wide DNA methylation sequencing, as well as RNA-seq on these purified populations, and our preliminary analysis reveals progressive and specific changes from 1 stage to the next (our unpublished data). The purified cells could also be used for screening drugs that would specifically act on a distinct erythroid stage that could, in turn, lead to novel therapeutic approaches for patients with altered erythropoiesis. Indeed, while it is known that dexamethasone and lenalidomide act on BFU-E and CFU-E cells, respectively,30 few drugs have been identified yet that would specifically act on any late-stage erythroblasts. Another relevant application for these purified populations would be to study enucleation using pure orthochromatic erythroblasts. To date, many studies on enucleation have used mixed populations of murine or human erythroblasts.31-34 In those studies, it is difficult to dissect out the effect of genetic or chemical manipulation on proliferation, differentiation, or enucleation. Because purified orthochromatic erythroblasts lack proliferation capability, they provide a powerful resource for studying the enucleation process per se.
Most importantly, for the first time, the combination of GPA, band 3, and α4 integrin have enabled us to purify human erythroblasts at every distinct stage of development from primary human bone marrow to quantify in vivo human terminal erythroid differentiation. Moreover, application of this novel method to MDS revealed an altered terminal erythroid differentiation process in MDS patients. As MDS syndromes are a heterogeneous group of disorders with 6 subtypes, according to classifications of the World Health Organization,35 future studies are needed to examine whether there are correlations between terminal erythropoiesis profiles and MDS subtypes. Changes in the terminal erythropoiesis profile might also be used to monitor disease progression, as well as response to drug treatment.
In summary, we systematically examined the changes of red cell membrane proteins during human terminal erythroid differentiation. The findings led to the identification of surface markers that enabled separation of distinct stages of erythroblasts from both in vitro differentiated erythroid cells and from primary human bone marrow. We suggest that use of these markers can monitor the erythropoiesis process in vitro and study altered erythropoiesis in vivo in various human erythroid disorders.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported in part by grants from the National Institutes of Health (DK26263 and DK32094).
Authorship
Contribution: J.H., J.L., F.X., A.G., and L.C. performed experiments and analyzed the data; G.H. and M.R. provided the valuable antibodies; A.R. and N.G. provided MDS patient human bone marrow samples and contributed to analysis of MDS samples; J.J. and J.L. provided normal human bone marrow samples; J.A.C., N.T., and N.M. designed the experiments, analyzed the data, and edited the manuscript; and X.A. designed the experiments, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xiuli An, Laboratory of Membrane Biology, 310 E 67th Street, New York, NY 10065; e-mail: xan@nybloodcenter.org.
References
Author notes
J.H. and J.L. contributed equally to this study.