Key Points
IL-12Rβ1–deficient subjects displayed substantially less circulating memory Tfh and memory B cells than control subjects.
The IL-12–STAT4 axis is associated with the development and functions of Tfh cells in vivo in humans.
Abstract
Antibody responses represent a key immune protection mechanism. T follicular helper (Tfh) cells are the major CD4+ T-cell subset that provides help to B cells to generate an antibody response. Tfh cells together with B cells form germinal centers (GCs), the site where high-affinity B cells are selected and differentiate into either memory B cells or long-lived plasma cells. We show here that interleukin-12 receptor β1 (IL-12Rβ1)–mediated signaling is important for in vivo Tfh response in humans. Although not prone to B cell-deficient–associated infections, subjects lacking functional IL-12Rβ1, a receptor for IL-12 and IL-23, displayed substantially less circulating memory Tfh and memory B cells than control subjects. GC formation in lymph nodes was also impaired in IL-12Rβ1–deficient subjects. Consistently, the avidity of tetanus toxoid–specific serum antibodies was substantially lower in these subjects than in age-matched controls. Tfh cells in tonsils from control individuals displayed the active form of signal transducer and activator of transcription 4 (STAT4), demonstrating that IL-12 is also acting on Tfh cells in GCs. Thus, our study shows that the IL-12–STAT4 axis is associated with the development and the functions of Tfh cells in vivo in humans.
Introduction
T follicular helper (Tfh) cells are essential for the generation of high-affinity memory B cells through the germinal center (GC) reaction.1-3 Tfh cells express the chemokine (C-X-C) receptor 5 (CXCR5),4-7 which guides their migration into B-cell follicles. Inducible costimulator (ICOS), expressed at high density by Tfh cells in human tonsils,7 plays a critical role for their development8-10 and function.11,12 Tfh cells support the differentiation and survival of GC B cells13,14 through the secretion of interleukin (IL)-21.15,16 Tonsillar Tfh cells express the transcription repressor B-cell lymphoma 6 (Bcl-6) at higher levels than any other CD4+ T-cell subsets.7,16-18 Mouse studies indicate that Bcl-6 is critical for Tfh cell generation in vivo, whereas Blimp-1, the transcription repressor that suppresses Bcl-6 function, inhibits their generation.19-21 In addition to GC response, CD4+ T cells also provide help to B cells at extrafollicular sites and induce their differentiation into plasma cells that contribute to the early generation of specific antibodies after antigen challenge.22 Extrafollicular helper cells appear to share the developmental mechanisms, phenotypes, and functional properties with Tfh cells.16,23-25
In mice, signal transducer and activator of transcription 3 (STAT3) signaling delivered by cytokines such as IL-6 and IL-21 contributes to the development of Tfh lineage cells.1 Also in humans, IL-6 and IL-21 can induce in vitro human naïve CD4+ T cells to express IL-21.18,26 IL-23, another STAT3-activating cytokine, also induces in vitro human CD4+ T cells to express some IL-21.18,26 Human STAT3-deficient subjects (Hyper IgE syndrome) display altered Tfh responses, which provides evidence that STAT3 signaling contributes to the generation of Tfh cells also in humans.27
In vitro studies with human cells suggested a role of the IL-12–STAT4 pathway in the commitment of naïve CD4+ T cells into the Tfh lineage. IL-12 induces human naïve CD4+ T cells to express IL-21 more potently than IL-6 and IL-21.18,26 The IL-12–STAT4 pathway also contributes to the expression of Tfh-associated molecules in mouse CD4+ T cells,28,29 although this effect appears to be short lived.28 Thus, both STAT3 and STAT4 signaling appears to be involved in the generation of Tfh cells in mice and humans. However, the contribution of each pathway and/or each cytokine might be different between the two species. In particular, whether the IL-12–STAT4 axis contributes to in vivo Tfh and GC responses in humans remains to be addressed.
IL-12 and IL-23 require a common receptor molecule, IL-12Rβ1, for high-affinity binding.30 IL-12Rβ1 deficiency is the most common genetic etiology of Mendelian susceptibility to mycobacterial disease, such as dissemination of Bacille Calmette-Guérin (BCG) after vaccination, as >100 cases with various IL12RB1 gene mutations have been identified.31,32 T cells from these subjects do not express functional IL-12Rβ1, and accordingly, completely lack the capacity to respond to IL-12 and IL-23.31,32 IL-12Rβ1–deficient subjects display impaired generation of interferon (IFN)-γ and IL-17–producing T cells and are susceptible to weakly pathogenic mycobacteria (including BCG), Salmonella, and Candida.31,33
Herein we determined the significance of IL-12Rβ1–mediated signaling in human Tfh and GC response in vivo by analyzing blood and lymph node (LN) samples from subjects lacking IL-12Rβ1. We show that IL-12Rβ1–deficient subjects displayed substantially less circulating memory Tfh and memory B cells than control subjects. GC formation in LNs was also impaired in IL-12Rβ1–deficient subjects. Furthermore, the avidity of tetanus toxoid (TT)–specific serum antibodies was substantially lower in these subjects than in age-matched controls. We also provide evidence that the IL-12–STAT4 axis is associated with the development and function of Tfh cells in vivo in humans.
Materials and methods
Clinical samples
Blood samples were obtained from IL-12Rβ1–deficient and healthy control subjects. Serum samples were stored at −20°C. Peripheral blood mononuclear cells (PBMCs) were isolated by centrifugation on a gradient (Ficoll-Paque; Stemcell), frozen, and stored in liquid nitrogen. LN samples were obtained from IL-12Rβ1–deficient children for diagnostic purposes of lymphadenopathy after BCG vaccinations. Pathological examinations showed the presence of bacilli, confirming the BCG infection. Obtained LNs were embedded in paraffin. The size of obtained LNs was ∼2 to 3 cm in diameter for each LN. The study was approved by the Institutional Review Boards of Baylor Health Care System and Necker Medical School, University Paris. Informed consent was obtained from subjects, parents, or legal guardians in accordance with the Declaration of Helsinki.
Flow cytometry
For surface staining, cells were incubated with the indicated antibodies and LIVE/DEAD fixable Aqua (Invitrogen) for 20 minutes at room temperature in phosphate-buffered saline. For the analysis of intracytoplasmic IL-21 expression, cultured CD4+ T cells were stimulated with phorbol 12-myristate 13-acetate (PMA; 25 ng/mL) and ionomycin (1 μg/mL) for 6 hours in the presence of GolgiStop (BD Biosciences) and Brefeldin (eBioscience) for the last 4 hours before staining. These reagents included the following: CXCR5 (1G10), CD3 (UCHT1), CD8 (SK1), CD4 (RPA-T4), IgD (IA6-2), CD27 (M-T271), CD212 (2.4E6), IL-21 (3A3-N2) monoclonal antibodies (mAbs), and streptavidin were from BD. CD8 (RPA-T8), HLA-DR (L243), CD20 (2H7), CD56 (HCD56), and IFN-γ (4S.B3) mAbs were from Biolegend. CD45RA (2H4) and CD19 (J3-119) mAbs were from Beckman Coulter. CD45 (HI30) mAbs were from Invitrogen. ICOS (ISA-3) mAb was purchased from eBioscience. CCR7 (150503) was from R&D systems. Bcl-6 expression was determined using anti-Bcl6 mAb from BD (K112-91) according to manufacturer’s recommendations. Cells were acquired on a BD LSRII. Expression of each molecule was assessed with FlowJo software (TreeStar).
Cytokine secretion
Frozen/thawed PBMCs (2.5 × 105 cells/well) were cultured in RPMI 1640 complete medium containing 10% fetal calf serum in the presence of Staphylococcal enterotoxin B (SEB) (0.1 μg/mL; Toxin Technology) in 96 round-bottom plates. Anti–IL-12p70 (20C2; Pierce) blocking mAb or IL-12 (10 ng/mL; R&D Systems) was added as indicated in some experiments. Supernatants were harvested after 48-hour culture, and cytokine levels were assessed by Luminex technology.
Immunohistochemistry
Immunohistochemistry was performed on paraffin sections (1 μm thick). Antibodies against CD4 (4B12, RTU), Bcl-6 (PG-B6p, RTU), CD20 (L26, 1:300), CD3 (rabbit polyclonal, 1:200), and CD138 (MI15, RTU) were purchased from Dako. CD57 antibody (NK-1, 1:50 dilution) and IgM, IgG, and IgA antibodies (rabbit polyclonal) were from Cell Marque. Activation-induced cytidine deaminase (AID) antibody (rat mAb, EK2 5G9, 1:200) was from Cell Signaling. Biotin goat anti-rat IgG was from Jackson ImmunoResearch. The iVIEW DAB detection kit (Ventana) was used as a detection system. Slides images were acquired using a Nikon DXM1200C digital camera and an Olympus BX60 microscope with Plan 4×/0.13, 10×/0.30, 20×/0.50, 40×/0.75, and 100×/1.40 oil objectives.
Immunofluorescence
Six-micrometer frozen sections from tonsils fixed with cold acetone were labeled with 4 µg/mL of phosphorylated (p)-STAT4 (E-2; Santa Cruz) mAb, followed by anti-mouse IgG conjugated to A568. Sections were then stained with biotin-conjugated CD4 mAb (RPA-T4; Biolegend), followed by AF488 streptavidin. Finally, sections were counterstained for 2 minutes with 3 µM of the nuclear stain 4,6 diamidino-2-phenylindole (DAPI). To confirm specificity of p-STAT4 staining, anti-pSTAT4 antibody was preincubated with blocking peptide (200 μg/mL) for 30 minutes at room temperature. Slides were mounted with Fluoromount-G (Southern Biotech) and observed using Metamorph software version 6.2 (Universal Imaging Corporation), a Roper coolsnap HQ camera, and an Olympus BCXCR51 microscope with a Plan10×/0.40 objective. Slides were also observed under a Leica SP5 confocal microscope with 20×/0.7, 40×/1.25, and 63×/1.4 Planapo objectives.
Enzyme-linked immunosorbent assay
Serum specific IgG titers and their avidities were determined using specific IgG enzyme-linked immunosorbent assay kits (IBL) according to the manufacturer’s protocol. For the measurement of avidities, the serum samples were incubated for 1 hour at room temperature in the enzyme-linked immunosorbent assay plates coated with the appropriate antigens. Then plates were washed 3 times with the provided wash buffer with or without 8 M urea, followed by 3 washes with the wash buffer. The avidity index was determined as the ratio of absorbance after incubation with urea to absorbance after incubation without urea and was expressed as a percentage.
Isolation of CD4+ T cells
PBMCs were purified from apheresis blood samples obtained from adult volunteers. Naïve CD4+ T cells were first enriched by negative selection with purified CD8 (HIT8a), CD11b (LM1/2), CD11c (B-ly6), CD14 (M5E2), CD15 (W6D3), CD16 (3G8), CD19 (J4.119), CD45RO (UCHL1), CD56 (C218), and HLA-DR (B8.12.2) mAbs and Dynabeads Pan Mouse IgG (Dynal). Naive CD4+ T cells were further purified by sorting with FACSAria (BD Biosciences) as CD4+CCR7+CD45RA+CD8−CD56−HLA-DR− cells. Naive CD4+ T cells (CCR7+CD56−CD8−CD45RA+HLA-DR−CD4+) and memory CD4+ T cells (CD56−CD8−CD45RA−CD4+) from an IL-12Rβ1 patient and 2 healthy controls were sorted on a FACS ARIA (BD). Cell purity was >99%.
Culture of CD4+ T cells
After overnight stimulation with CD3/CD28 Dynabeads (Invitrogen), cells were cultured in flat-bottomed 96-well plates coated with CD3 mAb (5 μg/mL, OKT3) and supplemented with soluble CD28 mAb (1 μg/mL, CD28.2) and the indicated cytokines (10 ng/mL each) or the supernatant of dendritic cells stimulated with CD40L-transfected L cells.
Nanostring
Naïve CD4+ T cells cultured for 48 hours with the indicated cytokines were lysed in RLT buffer. Total RNA was purified using the RNeasy Micro Kit (Qiagen). The NanoString reactions were performed according to the manufacturer’s instructions. The data were normalized to housekeeping genes included in the code set.
Tonsillar cells
Tonsil samples were obtained from healthy subjects undergoing tonsillectomies, and single cells were collected by mechanical disruption. B cells were positively selected with CD19 MACS Microbeads (Miltenyi Biotech), and GC B cells were sorted as CD3−CD20+IgD−CD38+ cells. Tfh were sorted as CD8−CD19−CD56−CD4+ICOShiCXCR5hi. GC B cells and GC Tfh cells (5 × 104 each/well) were cultured in 96-well round-bottom plates in the presence of 1 μg/mL SEB. Recombinant IL-12 (0.05 or 0.5 ng/mL), IL-23 (10 ng/mL), IFN-α2b (500 IU/mL), IFN-β (10 ng/mL), or IFN-ω (10 ng/mL) was added as indicated.
Statistics
The unpaired Student t test or nonparametric test was used. The paired Student t test was used in the analysis of IL-21 secretion by SEB-stimulated PBMCs in the presence or absence of IL-12 supplementation or IL-12 blocking mAbs. A Student t test with a 0.05 level of significance was used to determine whether parameter estimates were statistically significant.
Results
IL-12 and IL-23 induce naïve CD4+ T cells to express Tfh molecules
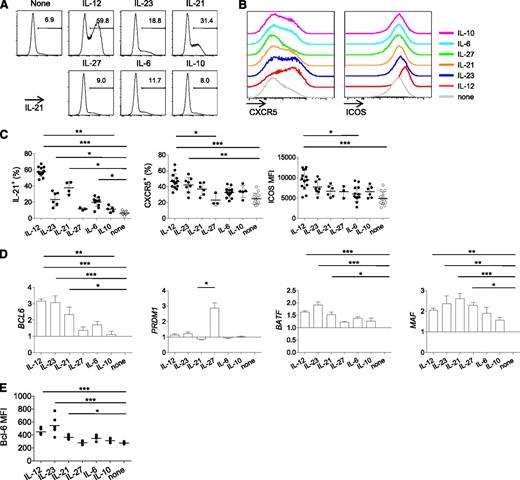
Previous in vitro studies have shown that IL-12 induces human naïve CD4+ T cells to express several molecules expressed by Tfh cells, such as IL-21, CXCR5, ICOS, and Bcl-6.18,26 To determine whether IL-12 is more potent than other cytokines in the induction of multiple Tfh-associated molecules, human naïve CD4+ T cells were stimulated for 3 days by CD3 and CD28 mAb-coated beads in the presence of IL-12, IL-23, IL-6, IL-21, IL-10, and IL-27. Cultured cells were analyzed for the expression of IL-21 (after 6-hour restimulation with PMA and ionomycin), CXCR5, and ICOS. Consistent with previous studies,18,26 IL-12 was the most potent among the tested cytokines at inducing primed naïve CD4+ T cells to express these three molecules (Figure 1A-C). We found that IL-23 also promoted IL-21 expression and CXCR5 expression. The analysis of the expression of transcription factors (TFs) by Nanostring showed that IL-12 and IL-23 were the most efficient at inducing the expression of Bcl-6 transcript (Figure 1D). Induction of Bcl-6 by IL-12 and IL-23 was confirmed at a protein level by flow cytometry (Figure 1E). In contrast, neither IL-12 nor IL-23 strongly promoted the expression of Blimp-1 (encoded by PRDM1; Figure 1D). IL-12 and IL-23 were also found to promote the expression of Batf, a TF essential for optimal Tfh cell generation.34 The expression of c-MAF, another TF that contributes to Tfh cell generation,35,36 was promoted by multiple cytokines including IL-12 and IL-23. Of note, IL-21 also induced naïve CD4+ T cells to express several Tfh-associated molecules, such as IL-21, Bcl-6, Batf, and c-Maf.
IL-12 and IL-23 induce naïve CD4+ T cells to express multiple Tfh-associated molecules. Human naïve CD4+ T cells were stimulated with CD3-CD28 mAb-coated beads in the presence of the indicated cytokines to analyze expression of Tfh-associated molecules. (A-C) Expression of IL-21 (after 6-hour restimulation with PMA and ionomycin), CXCR5, and ICOS on day 3. (A) A representative result of IL-21 expression. (B) A representative result of CXCR5 and ICOS expression. (C) Each dot represents data obtained from different donors. Mean ± standard deviation (SD); n = 3-13. One-way ANOVA: *P < .05; **P < .01; and ***P < .001. (D) Expression of BCL6, PRDM1, BATF, and MAF transcripts of CD4+ T cells cultured for 2 days. Abundance of transcripts was measured by Nanostring. Normalized to the abundance in the control conditions following gene normalization with 7 housekeeping genes. Mean ± SD; n = 3. One-way ANOVA. (E) Analysis of Bcl-6 expression by flow cytometry. CD4+ T cells were primed for 3 days in the presence of indicated cytokines. Mean ± SD; n = 3. One-way ANOVA.
IL-12 and IL-23 induce naïve CD4+ T cells to express multiple Tfh-associated molecules. Human naïve CD4+ T cells were stimulated with CD3-CD28 mAb-coated beads in the presence of the indicated cytokines to analyze expression of Tfh-associated molecules. (A-C) Expression of IL-21 (after 6-hour restimulation with PMA and ionomycin), CXCR5, and ICOS on day 3. (A) A representative result of IL-21 expression. (B) A representative result of CXCR5 and ICOS expression. (C) Each dot represents data obtained from different donors. Mean ± standard deviation (SD); n = 3-13. One-way ANOVA: *P < .05; **P < .01; and ***P < .001. (D) Expression of BCL6, PRDM1, BATF, and MAF transcripts of CD4+ T cells cultured for 2 days. Abundance of transcripts was measured by Nanostring. Normalized to the abundance in the control conditions following gene normalization with 7 housekeeping genes. Mean ± SD; n = 3. One-way ANOVA. (E) Analysis of Bcl-6 expression by flow cytometry. CD4+ T cells were primed for 3 days in the presence of indicated cytokines. Mean ± SD; n = 3. One-way ANOVA.
Thus, IL-12 and IL-23 strongly induce human naïve CD4+ T cells to express molecules important for the generation and function of Tfh cells.
IL-12Rβ1–deficient subjects display less CXCR5+ CD4+ T cells in blood
Human peripheral blood contains memory CD4+ T cells that express CXCR5, which likely represent a circulating memory compartment of Tfh cells.37,38 Consistently, subjects who show severely impaired GC formation through deficiency of CD40 ligand or ICOS display substantially fewer circulating CXCR5+CD4+ T cells.9 We analyzed the frequency of CXCR5+CD4+ T cells in PBMC samples obtained from IL-12Rβ1–deficient subjects (n = 22; age, 1-35 years) and healthy controls (n = 68; age, 2-35 years; supplemental Table 1 on the Blood Web site). In contrast to a recent study that observed no differences in the frequency of CXCR5+CD4+ T cells in blood,27 we found that IL-12Rβ1–deficient subjects displayed a lower frequency of CXCR5+ cells within total CD4+ T cells than did control subjects (Figure 2A-B, left; IL-12Rβ1 deficiency, 2.96 ± 0.50% [mean ± standard error of the mean], n = 22 vs control 7.48 ± 0.34%, n = 68). Decreased CXCR5+CD4+ T cells were not simply caused by a reduced generation of memory CD4+ T cells in IL-12Rβ1–deficient subjects (Figure 2B, center), as the frequency of CXCR5+ cells within memory CD4+ T cells was also substantially lower (Figure 2B, right). The intensity of CXCR5 expression on CXCR5+CD4+ T cells was also significantly lower in IL-12Rβ1–deficient subjects (Figure 2A,C).
IL-12Rβ1–deficient subjects display less CXCR5+ CD4+ T cells in blood. (A) Representative results of CXCR5 expression on blood CD4+ T cells. (B) Percentage of CXCR5+ cells within (left) total or (right) memory CD4+ T cells. Percentage of memory cells within total CD4+ T cells is shown in the center. Each dot represents a sample from either control subjects (n = 68, blue) or IL-12Rβ1–deficient subjects (n = 22, orange). Unpaired Student t test. (C) Mean fluorescent intensity of CXCR5 of CXCR5+ CD4+ T cells. (D) Percentage of CXCR5+ cells within (left) total and (right) memory CD4+ T cells at different ages. Each dot represents a sample from an individual subject. Linear regression and 95% confidence interval in each group (control or IL-12Rβ1 deficient) are indicated by solid lines and dotted lines, respectively. (E) Percentages of CXCR5+ cells within CD45RA−CD4+ T cells in groups of age <10. (F) CXCR5 expression in IL-12Rβ1–deficient subjects with different BCG vaccination/dissemination history. D, BCG disseminated; NV, not BCG vaccinated; R, subjects who remained asymptomatic (resistant) after BCG vaccination.
IL-12Rβ1–deficient subjects display less CXCR5+ CD4+ T cells in blood. (A) Representative results of CXCR5 expression on blood CD4+ T cells. (B) Percentage of CXCR5+ cells within (left) total or (right) memory CD4+ T cells. Percentage of memory cells within total CD4+ T cells is shown in the center. Each dot represents a sample from either control subjects (n = 68, blue) or IL-12Rβ1–deficient subjects (n = 22, orange). Unpaired Student t test. (C) Mean fluorescent intensity of CXCR5 of CXCR5+ CD4+ T cells. (D) Percentage of CXCR5+ cells within (left) total and (right) memory CD4+ T cells at different ages. Each dot represents a sample from an individual subject. Linear regression and 95% confidence interval in each group (control or IL-12Rβ1 deficient) are indicated by solid lines and dotted lines, respectively. (E) Percentages of CXCR5+ cells within CD45RA−CD4+ T cells in groups of age <10. (F) CXCR5 expression in IL-12Rβ1–deficient subjects with different BCG vaccination/dissemination history. D, BCG disseminated; NV, not BCG vaccinated; R, subjects who remained asymptomatic (resistant) after BCG vaccination.
Although reduced CXCR5 expression on CD4+ T cells was observed across all ages in IL-12Rβ1–deficient subjects (Figure 2D, left), the composition of CXCR5+ cells within memory CD4+ T cells was considerably reduced in <10-year-old children among IL-12Rβ1–deficient subjects (Figure 2D, right, E). The altered Tfh response in IL-12Rβ1–deficient children was not secondary to BCG infection and/or treatment, as there was no clear association between the frequency of CXCR5+CD4+ T cells and history of BCG vaccination/dissemination (Figure 2F).
Taken together, these data suggest that IL-12Rβ1–mediated signaling contributes to the generation and/or maintenance of Tfh responses. Decreased CXCR5 expression on memory CD4+ T cells was more apparent in IL-12Rβ1–deficient children, suggesting that this signaling is particularly important for Tfh responses during an early stage of life.
IL-12Rβ1–deficient subjects display less memory B cells in blood
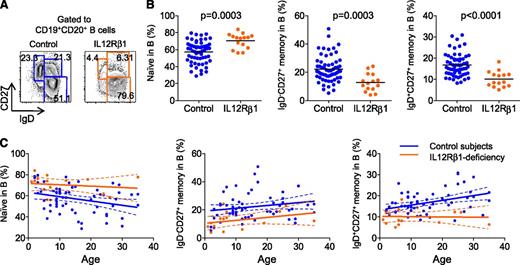
IL-12Rβ1–deficient subjects are not prone to B cell-deficient–associated infections.31 However, IL-12Rβ1–deficient subjects displayed a significantly higher proportion of IgD+CD27− naïve B cells across all ages (Figure 3A-C; IL-12Rβ1 deficiency, 70.39 ± 2.25% [mean ± standard error of the mean], n = 15 vs control, 57.10 ± 1.62%, n = 61). Accordingly, the proportion of IgD−CD27+ switched memory B cells and IgD+CD27+ nonswitched memory B cells was significantly lower in IL-12Rβ1–deficient subjects across all ages (IgD−CD27+: IL-12Rβ1 deficiency, 12.72 ± 1.62% vs control, 22.16 ± 1.18%; IgD+CD27+: IL-12Rβ1 deficiency, 10.13 ± 0.95% vs control, 16.75 ± 0.68%). These data show that the generation of memory B cells, their survival, or both events are altered in IL-12Rβ1–deficient subjects.
IL-12Rβ1–deficient subjects display less memory B cells in blood. (A) Representative results of phenotypic analysis of blood B-cell subsets. (B) Frequency of naïve (IgD+CD27−), IgD−CD27+ memory, and IgD+CD27+ nonswitched memory cells within CD20+ B cells in control subjects (n = 61, blue) or IL-12Rβ1–deficient subjects (n = 15, orange). Unpaired Student t test. (C) Composition of blood B-cell subsets at different ages. Each dot represents a sample from an individual subject. Linear regression and 95% confidence interval in each group (control or IL-12Rβ1 deficient) are indicated by solid lines and dotted lines, respectively.
IL-12Rβ1–deficient subjects display less memory B cells in blood. (A) Representative results of phenotypic analysis of blood B-cell subsets. (B) Frequency of naïve (IgD+CD27−), IgD−CD27+ memory, and IgD+CD27+ nonswitched memory cells within CD20+ B cells in control subjects (n = 61, blue) or IL-12Rβ1–deficient subjects (n = 15, orange). Unpaired Student t test. (C) Composition of blood B-cell subsets at different ages. Each dot represents a sample from an individual subject. Linear regression and 95% confidence interval in each group (control or IL-12Rβ1 deficient) are indicated by solid lines and dotted lines, respectively.
GC formation is altered in LNs of IL-12Rβ1–deficient subjects
As GCs are essential for the generation of memory B cells, we next determined whether GCs were present in inflammatory peripheral LNs obtained from 3 IL-12Rβ1–deficient children (called subjects 1-3). The LN samples were obtained from the IL-12Rβ1–deficient patients who showed massive LN enlargement after BCG vaccination (approximately 2-3 cm in diameter). Pathological examination of the samples showed the presence of bacilli, suggesting the infection by BCG.
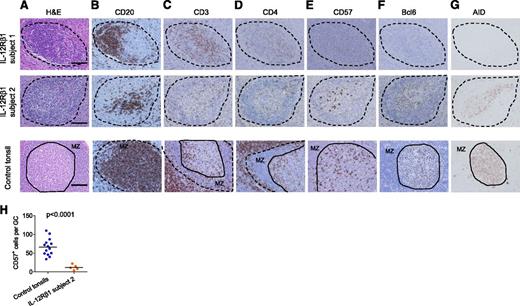
The 3 subjects presented different alterations of the lymphoid structures. Lymphoid aggregates were present in LNs from subjects 1 and 2 (Figure 4A; low-magnification images are shown in supplemental Figure 1). In LNs from subject 3, lymphoid aggregates were absent, which was likely associated with intensive inflammation (supplemental Figure 1). Lymphoid aggregates in the LNs from subjects 1 and 2 contained CD20+ B cells (Figure 4B) and some CD4+ T cells (Figure 4C-D; higher-magnification images are shown in supplemental Figure 2) and therefore displayed a characteristic of secondary follicles. Distinct from normal tonsillar GCs, however, no cells in the B-cell follicles from subject 1 expressed CD57 (Figure 4E). B cells in aggregates from subject 1 did not express detectable levels of AID, an enzyme essential for class switching and somatic hypermutation (Figure 4G, top).39 No Bcl-6hi cells were found in any secondary follicles in the LNs from subject 1 (Figure 4F, top), which further confirms the immaturity of GCs. In subject 2, some CD57+ cells were found in the T-B cell aggregates (Figure 4E). Furthermore, these T-B cell aggregates contained Bcl-6hi cells (Figure 4F) expressing detectable levels of AID (Figure 4G). However, the number of CD57+ cells per T-B cell aggregates was significantly lower than that in tonsillar GCs with a similar size (Figure 4H), indicating that the magnitude of GC response was substantially diminished in the LNs from subject 2. Moreover, distinct from normal GCs, the lymphoid mantle surrounding Bcl-6hi cell aggregates did not express CD20 and thus was not composed of B cells (Figure 4B). This suggests that the formation process of T-B cell aggregates was altered. Collectively, T-B cell aggregates were observed in LNs from two IL-12Rβ1–deficient subjects, but GC response was absent or diminished. This was unlikely because of overwhelming BCG accumulation at the sites and because no bacilli were found around the T-B cell aggregates (supplemental Figure 3).
IL-12Rβ1–deficient subjects altered GC responses. Alteration of GC formation in IL-12Rβ1–deficient subjects. Immunohistochemistry staining of peripheral LN samples from IL-12Rβ1–deficient subjects and tonsil samples from control subjects. Samples were stained with (A) H&E, (B) CD20 mAb, (C) CD3 polyclonal Ab, (D) CD4 mAb, (E) CD57 mAb, (F) Bcl-6 mAb, and (G) AID mAb. T-B cell aggregates in subjects 1 and 2 are indicated by dotted lines. In control tonsils, secondary follicles are indicated by dotted lines, and the solid lines indicate the border between the mantle zone (MZ) and the GC. Bar equals 100 μm. (H) CD57+ cells per T-B cell aggregates. CD57+ cells were counted within the T-B cell aggregates in subject 2 LNs, and within GCs with a similar size present in tonsils from 3 control subjects. Student t test.
IL-12Rβ1–deficient subjects altered GC responses. Alteration of GC formation in IL-12Rβ1–deficient subjects. Immunohistochemistry staining of peripheral LN samples from IL-12Rβ1–deficient subjects and tonsil samples from control subjects. Samples were stained with (A) H&E, (B) CD20 mAb, (C) CD3 polyclonal Ab, (D) CD4 mAb, (E) CD57 mAb, (F) Bcl-6 mAb, and (G) AID mAb. T-B cell aggregates in subjects 1 and 2 are indicated by dotted lines. In control tonsils, secondary follicles are indicated by dotted lines, and the solid lines indicate the border between the mantle zone (MZ) and the GC. Bar equals 100 μm. (H) CD57+ cells per T-B cell aggregates. CD57+ cells were counted within the T-B cell aggregates in subject 2 LNs, and within GCs with a similar size present in tonsils from 3 control subjects. Student t test.
IL-12Rβ1–deficient subjects display TT-specific antibodies with lower avidities
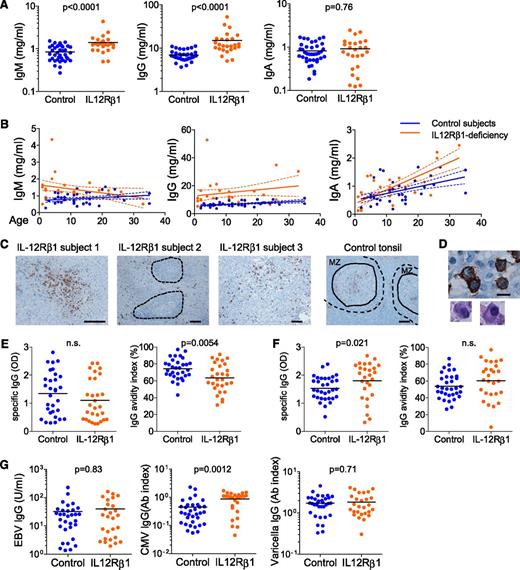
Given the alterations in Tfh and GC responses and in the generation of memory B cells, we hypothesized that IL-12Rβ1–deficient subjects might display lower serum Ig levels. Thus, serum IgM, IgG, and IgA levels were measured in 25 IL-12Rβ1–deficient subjects (age: 1-33 years old) and 38 age-matched controls (age: 2-35 years old). IL-12Rβ1–deficient subjects displayed higher serum IgM and IgG levels than healthy subjects (Figure 5A). In particular, serum IgG levels were higher across all ages (Figure 5B). This shows that class-switched plasma cells can be generated in IL-12Rβ1–deficient subjects. Indeed, CD138-expressing cells with typical plasma cell morphology were abundantly found in all three LNs, including LNs that lacked T-B cell aggregates (subject 3; Figure 5C-D). Both in subjects 1 and 2, CD138+ plasma cells were found exclusively outside T-B cell aggregates (Figure 5C). Consistently, Ig-expressing B cells were present in the three LNs, but were almost absent (in subject 1) or very few (in subject 2) within the T-B cell aggregates (supplemental Figure 4). These observations suggest that plasma cells might develop in IL-12Rβ1–deficient subjects mainly via extrafollicular mechanisms and/or T cell–independent mechanisms and that the contribution of the GC response might be limited.
IL-12Rβ1–deficient subjects display low-avidity TT-specific IgG. (A) Serum IgM, IgG, and IgA levels in IL-12Rβ1–deficient subjects (n = 25) and control subjects (n = 38). Student t test. (B) Serum Ig levels at different ages. Each dot represents a sample from an individual subject. Linear regression and 95% confidence interval in each group (control or IL-12Rβ1 deficient) are indicated by solid lines and dotted lines, respectively. (C) CD138 staining of peripheral LN samples from IL-12Rβ1–deficient subjects and tonsil samples from control subjects. T-B cell aggregates in subject 2 are indicated by dotted lines. In control tonsils, secondary follicles are indicated by dotted lines, and the solid lines indicate the border between the mantle zone (MZ) and the GC. Bar equals 100 μm. (D) CD138+ cells with plasma cell morphology. Bar equals 10 μm. (E) TT-specific IgG of serum samples were assessed by enzyme-linked immunosorbent assay. IgG avidity was assessed by the index calculated as a percentage: (OD after urea treatment/OD of the reference well) × 100. (F) Rubella virus–specific IgG levels and their avidities were assessed by enzyme-linked immunosorbent assay. (G) Serum IgG levels against Epstein-Barr virus, cytomegalovirus, and varicella virus in IL-12Rβ1–deficient subjects and control subjects. Student t test.
IL-12Rβ1–deficient subjects display low-avidity TT-specific IgG. (A) Serum IgM, IgG, and IgA levels in IL-12Rβ1–deficient subjects (n = 25) and control subjects (n = 38). Student t test. (B) Serum Ig levels at different ages. Each dot represents a sample from an individual subject. Linear regression and 95% confidence interval in each group (control or IL-12Rβ1 deficient) are indicated by solid lines and dotted lines, respectively. (C) CD138 staining of peripheral LN samples from IL-12Rβ1–deficient subjects and tonsil samples from control subjects. T-B cell aggregates in subject 2 are indicated by dotted lines. In control tonsils, secondary follicles are indicated by dotted lines, and the solid lines indicate the border between the mantle zone (MZ) and the GC. Bar equals 100 μm. (D) CD138+ cells with plasma cell morphology. Bar equals 10 μm. (E) TT-specific IgG of serum samples were assessed by enzyme-linked immunosorbent assay. IgG avidity was assessed by the index calculated as a percentage: (OD after urea treatment/OD of the reference well) × 100. (F) Rubella virus–specific IgG levels and their avidities were assessed by enzyme-linked immunosorbent assay. (G) Serum IgG levels against Epstein-Barr virus, cytomegalovirus, and varicella virus in IL-12Rβ1–deficient subjects and control subjects. Student t test.
Whichever case it is, one might expect antibodies to be low avidity. Thus, we analyzed the titer and avidity of serum antibodies specific for TT in IL-12Rβ1–deficient subjects. The avidity was analyzed by comparing the amount of antibodies that bound to TT-coated plates with and without treatment with urea, a treatment that that disrupts the low-avidity antigen-antibody complex.40,41 The IL-12Rβ1–deficient subjects showed TT-specific serum IgG levels comparable to those of age-matched control subjects (Figure 5E, left). However, their avidities were substantially lower (Figure 5E, right).
To address whether the generation of high avidity IgG is globally impaired in IL-12Rβ1–deficient subjects, we next analyzed the serum IgG titers and their avidities against rubella virus. IL-12Rβ1–deficient subjects displayed higher serum rubella-specific IgG levels than controls (Figure 5F, left). Furthermore, in contrast to TT-specific IgG, there was no difference in the avidities of specific IgG against rubella virus between IL-12Rβ1–deficient subjects and healthy subjects (Figure 5F, right). Furthermore, serum IgG titers against other viruses, including Epstein-Barr virus, cytomegalovirus, and varicella virus, were either normal or elevated in IL-12Rβ1–deficient subjects compared with those in healthy subjects (Figure 5G). Thus, generation of virus-specific antibody responses appears to be largely normal in IL-12Rβ1–deficient subjects. This is consistent with the observations that IL-12Rβ1–deficient subjects do not show susceptibility to viruses.31
Collectively, the selection process of high-affinity B cells against TT was altered in IL-12Rβ1–deficient subjects. In contrast, antibody responses against viruses were largely normal.
IL-21 secretion is impaired by IL-12Rβ1–deficient CD4+ T cells
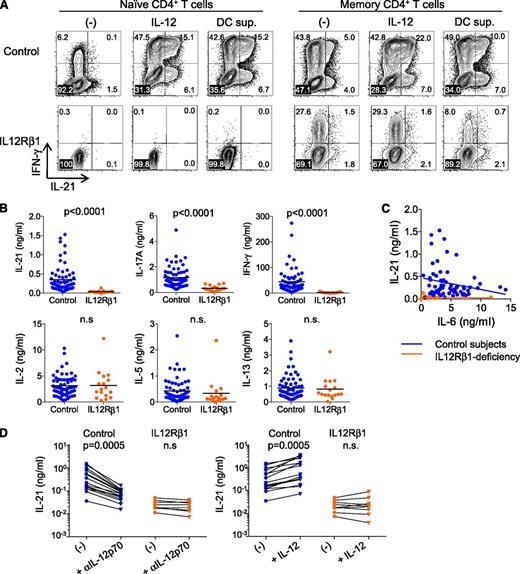
IL-21 is important for the up-regulation of Bcl-6 in B cells, hence the generation of GC B cells and GC persistence.13,14 IL-21 also contributes to human naïve CD4+ T cells to express Tfh-associated molecules (Figure 1). Therefore, the impaired Tfh and GC responses in IL-12Rβ1–deficient subjects might be associated with impaired secretion of IL-21 by CD4+ T cells. Indeed, naïve CD4+ T cells (CCR7+CD45RA+) or memory CD4+ T cells from an IL-12Rβ1–deficient subject (IL-12RB1-15; supplemental Table 1) did not express IL-21 following stimulation with CD3/CD28 mAbs—even in the presence of IL-12 or a proinflammatory cytokine-rich supernatant of CD40 ligand–stimulated dendritic cell (which included 23 ng/mL IL-6; Figure 6A).
Altered IL-21 secretion by IL-12Rβ1–deficient CD4+ T cells. (A) Intracytoplasmic cytokine expression by naïve and memory CD4+ T cells from an IL-12Rβ1–deficient subject and healthy subjects after 8-day culture with anti-CD3/CD28 and either IL-12 or CD40L-stimulated DC supernatant. (B) Cytokine secretion from PBMCs stimulated for 48 hours with SEB. Mann-Whitney test. Control subjects (blue), n = 65; IL-12Rβ1–deficient subjects (orange), n = 17. (C) Associations between IL-6 concentrations and IL-21 concentrations in culture supernatant of PBMCs stimulated with SEB. (D) Contribution of IL-12 in IL-21 secretion. An (left) IL-12p70 blocking antibody or (right) IL-12 (10 ng/ml) was added to the PBMC cultures with SEB. Paired Student t test. Control subjects (blue), n = 16; IL-12Rβ1–deficient subjects (orange), n = 8.
Altered IL-21 secretion by IL-12Rβ1–deficient CD4+ T cells. (A) Intracytoplasmic cytokine expression by naïve and memory CD4+ T cells from an IL-12Rβ1–deficient subject and healthy subjects after 8-day culture with anti-CD3/CD28 and either IL-12 or CD40L-stimulated DC supernatant. (B) Cytokine secretion from PBMCs stimulated for 48 hours with SEB. Mann-Whitney test. Control subjects (blue), n = 65; IL-12Rβ1–deficient subjects (orange), n = 17. (C) Associations between IL-6 concentrations and IL-21 concentrations in culture supernatant of PBMCs stimulated with SEB. (D) Contribution of IL-12 in IL-21 secretion. An (left) IL-12p70 blocking antibody or (right) IL-12 (10 ng/ml) was added to the PBMC cultures with SEB. Paired Student t test. Control subjects (blue), n = 16; IL-12Rβ1–deficient subjects (orange), n = 8.
Next, to analyze IL-21 production by blood CD4+ T cells, PBMCs from IL-12Rβ1–deficient subjects were stimulated for 48 hours with the superantigen SEB.26 As shown in Figure 6B, PBMCs from IL-12Rβ1–deficient subjects secreted much less IL-21 than PBMCs from healthy aged-matched controls (IL-12Rβ1 deficiency, 0.034 ± 0.01 ng/mL [mean ± standard deviation], n = 17 vs control, 0.35 ± 0.04 ng/mL, n = 65). The amounts of IL-6 in the supernatants of IL-12Rβ1–deficient cells (2.0 ± 2.9 ng/mL [mean ± standard deviation) did not correlate with the amounts of IL-21 (Figure 6C). This suggests that IL-6 contributed little to the secretion of IL-21 by IL-12Rβ1–deficient cells. As previously described,31,33 the secretion of IFN-γ and IL-17A was also lower in IL-12Rβ1–deficient subjects (Figure 6B). In contrast, IL-2 and type 2 cytokines (including IL-5 and IL-13) were secreted at equivalent levels in the 2 groups.
We hypothesized that impaired IL-21 secretion by IL-12Rβ1–>deficient cells was, at least partially, due to their inability to respond to IL-12 secreted by antigen-presenting cells. As shown in Figure 6D, IL-21 secretion by SEB-stimulated normal PBMCs decreased in the presence of IL-12 neutralizing mAb and increased in response to exogeneous IL-12. However, neither blocking nor addition of IL-12 modified IL-21 secretion in SEB-stimulated PBMCs from IL-12Rβ1–deficient subjects.
Thus, IL-21 secretion by CD4+ T cells from IL-12Rβ1–deficient subjects was impaired at least partly because of the inability to respond to IL-12. The contribution of IL-6 in the secretion of IL-21 by IL-12Rβ1–deficient CD4+ T cells was minimal.
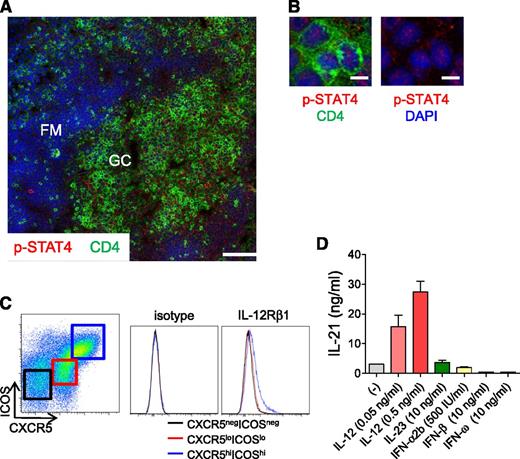
Tfh cells in normal tonsils display phosphorylated STAT4
The results in Figures 1 and 6D show that IL-12 is important for naïve and memory CD4+ T cells to produce IL-21. It is possible that IL-12 may also act on mature Tfh cells in GCs, as dendritic cells localized in GCs are known to produce IL-12.42 As IL-12 signaling is mediated by an active form of p-STAT4, we analyzed the expression of p-STAT4 in GC Tfh cells present in normal tonsils. A vast majority of tonsillar Tfh cells were found to express p-STAT4 translocated in nuclei (Figure 7A-B), which is evidence of active STAT4 signaling. Consistently, tonsillar GC Tfh cells (indicated by CXCR5hiICOShiCD4+ T cells16 ) expressed IL-12Rβ1 on the cell surface at higher levels than CXCR5−ICOS−CD4+ T cells (which mainly are composed of naïve T cells16 ) and CXCR5loICOSloCD4+ T cells (Figure 7C). Furthermore, tonsillar GC Tfh cells produced increased levels of IL-21 in response to IL-12 (Figure 7D). Notably, IL-21 secretion by tonsillar Tfh cells was not increased in response to other STAT4-activating cytokines such as IL-23 or type I IFNs (Figure 7D). These observations suggest that human Tfh cells in GCs receive local IL-12 signaling and enhance IL-21 secretion.
IL-12/STAT4 signaling in human tonsillar GC Tfh cells. (A) p-STAT4 expression by GC Tfh cells. Cells expressing p-STAT4 (red) and CD4 (green) in human tonsil were analyzed by confocal microscopy. Blue indicates DAPI staining. DZ, dark zone; FM, follicular mantle; LZ, light zone. Bar equals 100 μm. (B) Nuclear localization of p-STAT4 in Tfh cells is shown on the right with DAPI staining. Bar equals 10 μm. (C) Expression of IL-12Rβ1 on tonsillar GC Tfh (CXCR5hiICOShiCD4+ T) cells. (Left) Gating strategy for CXCR5−ICOS−, CXCR5loICOSlo, and CXCR5hiICOShi within CD3+CD4+ T cells. Representative results from 3 independent experiments. (D) IL-21 secretion by tonsillar GC Tfh cells in response to IL-12. Tonsillar Tfh cells were stimulated for 3 days with autologous SEB-pulsed B cells in the presence of IL-12, IL-23, or type I IFNs. Representative results from 3 independent experiments.
IL-12/STAT4 signaling in human tonsillar GC Tfh cells. (A) p-STAT4 expression by GC Tfh cells. Cells expressing p-STAT4 (red) and CD4 (green) in human tonsil were analyzed by confocal microscopy. Blue indicates DAPI staining. DZ, dark zone; FM, follicular mantle; LZ, light zone. Bar equals 100 μm. (B) Nuclear localization of p-STAT4 in Tfh cells is shown on the right with DAPI staining. Bar equals 10 μm. (C) Expression of IL-12Rβ1 on tonsillar GC Tfh (CXCR5hiICOShiCD4+ T) cells. (Left) Gating strategy for CXCR5−ICOS−, CXCR5loICOSlo, and CXCR5hiICOShi within CD3+CD4+ T cells. Representative results from 3 independent experiments. (D) IL-21 secretion by tonsillar GC Tfh cells in response to IL-12. Tonsillar Tfh cells were stimulated for 3 days with autologous SEB-pulsed B cells in the presence of IL-12, IL-23, or type I IFNs. Representative results from 3 independent experiments.
Discussion
Our present study provides in vivo evidence that IL-12Rβ1–mediated signaling is involved in human Tfh reactions. This conclusion is supported by four observations: (1) IL-12Rβ1–deficient subjects display a decreased frequency of CXCR5+CD4+ T cells in blood; (2) IL-12Rβ1–deficient subjects show a reduced frequency of class-switched IgD−CD27+ memory B cells in blood; (3) GC formation appears to be impaired in LNs of IL-12Rβ1–deficient subjects; and (4) IL-12Rβ1–deficient subjects display low-avidity TT-specific antibodies.
In vitro, IL-12 and IL-23 induced naïve CD4+ T cells to express Tfh molecules, such as IL-21, CXCR5, and ICOS, as well as multiple TFs important for Tfh cell generation, such as Bcl-6, c-Maf, and Batf. Although STAT3 signaling contributes to human Tfh cell generation,27 IL-12 and IL-23 were superior to IL-6 and IL-21 in the induction of Tfh-associated molecules. In vivo, IL-12Rβ1–deficient subjects displayed less CXCR5+CD4+ T cells in blood. Although a recent report concluded that IL-12Rβ1–deficient subjects display a normal frequency of CXCR5+CD4+ T cells in blood,27 such a discrepancy could be due to the differences in the subject ages between the 2 studies, because we observed that the reduction of blood CXCR5+CD4+ T cells was particularly profound in IL-12Rβ1–deficient children. In healthy children, the composition of blood CXCR5+CD4+ T cells rapidly increase during the first 6 months of life and peak at approximately 1 to 2 years of age.43 This is consistent with the fact that the splenic lymphoid structure matures during the first year.44 Thus, our data suggest that IL-12Rβ1–mediated signaling is also essential to mature the immune system for optimal humoral responses in the early stage of life.
Consistent with decreased CXCR5+CD4+ T cells in blood, GC formation appears to be impaired in LNs of IL-12Rβ1–deficient subjects. Among the 3 tested LNs, 1 did not contain any T-B cell aggregates (subject 3) and thus was excluded from the assessment of GC formation. Another contained multiple T-B cell aggregates but lacked cells expressing Bcl-6 (subject 1). The other contained T-B aggregates that contained CD57+ T cells and Bcl-6+AID+ B cells, but the number of CD57+ T cells per T-B aggregates was substantially lower than that of healthy tonsillar GCs (subject 2), indicating the attenuated GC-like responses. Impaired GC responses likely explain why IL-12Rβ1–deficient subjects displayed less memory B cells in blood. It is also possible that the lack of IL-12Rβ1–mediated signaling in B cells affect their differentiation and/or survival as well.45,46 Alternatively, the lack of IL-12Rβ1–mediated signaling might cause a preferential differentiation toward plasma cells to the expense of memory cells, as the signals required for their generation seem distinct.47,48
Nonetheless, IL-12Rβ1–deficient subjects were shown to generate antibody responses on vaccination or microbial infection.49-51 We found that serum IgG levels were higher in IL-12Rβ1–deficient subjects than in healthy subjects. These observations show that Ig-producing plasma cells can develop and can undergo class-switching independent of IL-12 or IL-23. Indeed, plasma cells were present in the LNs from IL-12Rβ1–deficient subjects, including the sample that did not contain any T and B cell aggregates (subject 3). Thus, Ig-producing plasma cells appear to develop in IL-12Rβ1–deficient subjects via an altered GC reaction and/or extrafollicular pathway. T-independent mechanisms might contribute as well. Generation of low-avidity antibodies against TT in IL-12Rβ1–deficient subjects supports this hypothesis. However, our study shows that the development of virus-specific antibody responses was almost intact in IL-12Rβ1–deficient subjects. First, IL-12Rβ1–deficient subjects showed normal or even higher serum virus-specific IgG titers than healthy subjects, including rubella virus, Epstein Barr virus, cytomegalovirus, and varicella virus. Second, the avidities of rubella-specific IgG were similar between IL-12Rβ1–deficient subjects and healthy subjects. Such differences in the quality of antibody responses in IL-12Rβ1–deficient subjects might depend on the mode of antigen delivery. TT vaccine is composed of tetanus toxin adjuvanted with alum (usually mixed with other toxins from diphtheria and pertussis). In contrast, rubella vaccine is composed of live attenuated virus, which might induce prolonged viral replication and thus antigen retention in IL-12Rβ1–deficient subjects, due to impaired generation of IFNγ-producing specific T cells. Similarly, natural viral infection might cause prolonged viral replication. Such prolonged viral retention might yield GC-like responses sufficient for the selection of high-affinity antibodies. Collectively, these observations show that, although Tfh response is impaired, IL-12Rβ1–deficient subjects still can mount normal antiviral antibody responses.
Last, our study also suggests that the IL-12–STAT4 axis plays a role in the function of Tfh cells. IL-12Rβ1–deficient CD4+ T cells secreted less IL-21 than normal CD4+ T cells, at least partly due to the inability to respond to IL-12. Furthermore, GC Tfh cells in normal tonsils display p-STAT4, indicating that GC Tfh cells locally receive IL-12 signaling. Consistently, GC Tfh cells show increased IL-21 secretion in response to IL-12. Because IL-21 is involved in the generation and the maintenance of GC reactions,13,14 impairment in the secretion of IL-21 is likely associated with altered GC response in IL-12Rβ1–deficient subjects. As IL-12 is potent at inducing CD4+ T cells to express CXCR5, the IL-12–STAT4 axis might also contribute to the maintenance of CXCR5 expression on GC Tfh cells. Notably, IL-12Rβ1–deficient subjects were not entirely deficient of Tfh cells because they were able to display near normal levels of blood CXCR5+CD4+ T cells on aging. This suggests that other pathways such as STAT3 signaling mediated by cytokines other than IL-12 or IL-23 can compensate the generation and/or maintenance of Tfh responses.
Our study will provide an implication in the design of novel vaccines for infectious diseases. Furthermore, our study suggests that dysregulated IL-12–STAT4 signaling might contribute to the pathogenesis of human autoimmune disease by promoting excessive Tfh and GC response. Blocking this pathway might be beneficial for the prevention and/or treatment of the diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the donors for providing samples for the study; E. Kowalski and S. Coquery for cell sorting; G. Zurawski and S. Zurawski for multiplex cytokine analysis; and R. Coffman, C. Harrod, G. Zurawski, A. K. Palucka, and Y. J. Liu for critical reading and discussions.
This study was supported by research funding from National Institutes of Health, National Institute of Allergy and Infectious Diseases, grants U19-AI057234, U19-AI082715, and U19-AI089987 and Baylor Health Care System.
Authorship
Contribution: N.S. and H.U. designed the study; N.S. performed most of the experiments; L.B. performed immunofluorescence staining; S.E.B. prepared tonsil samples; J. Bustamante, S.B.-D., and J.-L.C. provided patients and control samples and clinical information; F.H., M.V.T., and D.A.S. performed immunohistochemistry staining and contributed to the discussion; D.B. contributed the statistics; V.P. contributed to the design of some experiments; and N.S., J. Banchereau, J.-L.C., and H.U. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nathalie Schmitt, Baylor Institute for Immunology Research, 3434 Live Oak, Dallas, TX 75204; e-mail: nathalis@baylorhealth.edu; and Hideki Ueno, Baylor Institute for Immunology Research, 3434 Live Oak, Dallas, TX 75204; e-mail: hidekiu@baylorhealth.edu.